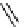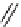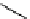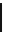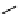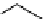Chemistry Reference
In-Depth Information
4.2.1
synthesis with [
11
c]carbon dioxide
11
CO
2
is the most widely produced in target C-11 primary precursor. Although it is often converted to other reactive secondary
precursors (scheme 4.1),
11
CO
2
is a versatile reagent in its own right and has been recently exploited for the rapid labelling
of a range of useful functional groups and target molecules. The main advantage of using
11
CO
2
directly is that labelling can
be performed much faster and without the need for time-consuming secondary precursor production. One of the drawbacks
of its direct reaction is the potential risk of contamination with atmospheric
12
CO
2
, which can diminish specific radioactiv-
ities; however, with due care, excessive contamination with atmospheric CO
2
may be minimised. Carbon dioxide is widely
known to rapidly react with organometallic reagents such as grignard and organolithium reagents. The reaction of
11
CO
2
with grignard reagents can be used to prepare a range of [
11
C]carboxyl acids from the corresponding grignards following
hydrolysis. [
Carbonyl
-
11
C]amides may be formed directly from [
11
C]carboxymagnesium halides using conventional heating
[4, 5] or microwave assisted heating [6] (scheme 4.2).
[
Carbonyl
-
11
C]acetate, which is used in the evaluation of myocardial oxygen metabolism [7] and diagnosis of prostate
cancer [8], can be conveniently produced via [
11
C]carboxylation of methyl magnesium bromide [9-11] following a quench-
ing and neutralisation step. [
11
C]carboxylic acids may be converted into more reactive acid chloride species via reaction with
chlorinating agents such as thionyl chloride or phthaloyl dichloride. This route has been used for the synthesis of [
carbonyl
-
11
C]amides and has been effectively applied to labelling the important 5HT
1A
receptor ligand WAy100635 (scheme 4.3) [12, 13]
and to the oncology biomarker BAy 59-8862, a derivative of taxane [14].
recently, boronic acid esters have been used for metal-mediated
11
CO
2
carboxylation reactions. in contrast to organomag-
nesium and organolithium reagents, boronic acid esters are stable toward air and moisture and, importantly, have a high
degree of functional group tolerance. riss et al. [15] demonstrated a highly effective way of performing Cui-mediated car-
boxylations of boronic acid esters using a combination of TMEDA, KF, and crypt-222 (scheme 4.4). A range of [
11
C]
carboxylic acids was obtained in high rCy that would be otherwise difficult to synthesise using traditional organometallic
O
O
O
H
+
/H
2
O
SOCl
2
11
CO
2
R M
*
R
O
M
R
*
OH
R
*
Cl
M = MgX or Li
HNR
ʹ
R
ʹʹ
conventional
heating or
microwave
heating
HNR
ʹ
R
ʹʹ
O
R
ʹʹ
*
R
N
R
ʹ
scheme 4.2
reaction of
11
CO
2
with organometallic reagents to form [
carbonyl
-
11
C]carboxylic acids, [
carbonyl
-
11
C]acid chlorides,
and [
carbonyl
-
11
C]amides. [*] indicates C-11 labelling position.
OCH
3
O
O
Cl
MgCl
N
N
*
1.
11
CO
2
WAY100364
*
N
2. HCl, THF
3. SOCl
2
NEt
3
, THF
N
[carbonyl-
11
C] WAY100365
scheme 4.3
One-pot synthesis of [
carbonyl
-
11
C]WAy100635 via reaction of [
carbonyl
-
11
C]cyclohexyl acid chloride with WAy100634.
[*] indicates C-11 labelling position.
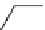
O
O
11
CO
2
, CuI, KF, crypt-222
TMEDA, DMF, 10 min
B
O
*
OH
scheme 4.4
Model reaction of phenyl boronic ester with
11
CO
2
to form [
carbonyl
-
11
C]benzoic acid in high rCy (99%). [*] indicates
C-11 labelling position.