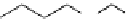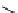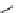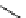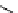Chemistry Reference
In-Depth Information
N
3
TsO
107
K[
18
F]F-K
222
, 80°C
distillation
O
O
OO
18
F
N
3
18
F
Ph
S
PPh
2
Ph
H
108
H
H
CH
3
CN / DMF / H
2
O
130°C, 15 min
106
109
scheme 3.19
Example of the synthesis of an
N
-(2-[
18
F]luoroethyl)amide (
109
) using the Staudinger ligation.
azide function is envisaged on the biomolecule, variant A of Scheme 3.18 has been chosen so far with R
1
=
p
-[
18
F]fluorophe-
nyl,
p
-(2-[
18
F]fluoroethyl)phenyl or 5-[
18
F]fluoropentyl [258, 260, 262]. The radiochemistry of these phosphine compounds
is not always straightforward, and reaction conditions such as solvents have to be selected carefully.
3.6
conclusIons
Fluorine-18 chemistry for PET has now reached a certain degree of maturity. Notably nucleophilic aliphatic and aromatic
substitution have been consolidated to robust and reliable methodologies serving as a stepping stone for further development.
The advent of microfluidic synthesis devices is promoting a nucleophilic radiofluorination in which drying is not necessarily
a prerequisite. In bioconjugation, new click-like procedures are following each other at a great pace while alumina, boron, and
silicon claim their place next to carbon as the reaction centre. In electrophilic substitution, after a long status quo, new ideas
have begun to emerge on how to keep the SRA high. Thus, the field of fluorine-18 chemistry continues to invite significant
creativity and thus should be assured of a bright future.
references
[1] S. Banister, D. Roeda, F. Dollé and M. Kassiou,
Curr. Radiopharm.
3
, 68‒80 (2010).
[2] N. N. Ryzhikov, N. Seneca, R. N. Krasikova, N. A. Gomzina, E. Shchukin, O. S. Fedorova, D. A. vassiliev, B. Gulyas, H. Hall,
I. Savic and C. Halldin,
Nucl. Med. Biol.
32
, 109‒116 (2005).
[3] F. Dollé, F. Hinnen, P. Emond, S. Mavel, Z. Mincheva, W. Saba, M.-A. Schöllhorn-Peyronneau, H. valette, L. Garreau, S. Chalon,
C. Halldin, J. Helfenbein, J. Legaillard, J.-C. Madelmont, J.-B. Deloye, M. Bottlaender and D. Guilloteau,
J. Label Compounds
Radiopharm.
49
, 687‒698 (2006).
[4] F. Dollé, J. Helfenbein, F. Hinnen, S. Mavel, Z. Mincheva, W. Saba, M.-A. Schöllhorn-Peyronneau, H. valette, L. Garreau,
S. Chalon, C. Halldin, J.-C. Madelmont, J.-B. Deloye, M. Bottlaender, J. Legaillard, D. Guilloteau and P. Emond,
J. Label.
Compounds Radiopharm.
50
, 716‒723 (2007).
[5] M. R. Zhang and K. Suzuki,
Curr. Topics Med. Chem.
7
, 1817‒1828 (2007).
[6] M. L. James, R. R. Fulton, J. vercoullie, D. J. Henderson, L. Garreau, S. Chalon, F. Dollé, S. Selleri, D. Guilloteau and M. Kassiou,
J. Nucl. Med.
49
, 814‒822 (2008).
[7] A. Damont, F. Hinnen, B. Kuhnast, M.-A. Schöllhorn-Peyronneau, M. L. James, C. Luus, B. Tavitian, M. Kassiou and F. Dollé,
J. Label. Compounds Radiopharm.
51
, 286‒292 (2008).
[8] B. Kuhnast, A. Damont, F. Hinnen, T. Catarina, S. Demphel, S. Le Helleix, C. Coulon, S. Goutal, P. Gervais and F. Dollé,
Appl.
Radiat. Isot.
70
, 489‒477 (2012).
[9] M. R. Kilbourn, B. Hockley, L. Lee, C. Hou, R. Goswami, D. E. Ponde, M-P. Kung and H. F. Kung,
Nucl. Med. Biol.
34
, 233‒237
(2007).
[10] L. Zhu, y. J. Liu, K. Plossl, B. Lieberman, J. y. Liu and H. F. Kung,
Nucl. Med. Biol.
37
, 133‒141 (2010).
[11] E. Hess, S. Takács, B. Scholten, F. Tárkányi, H. H. Coenen and S. M. Qaim,
Radiochim. Acta
89
, 357‒362 (2001).
[12] T. J. Ruth, K. R. Buckley, K. S. Chun, E. T. Hurtado, S. Jivan and S. Zeisler,
Appl. Radiat. Isot.
55
, 457‒461 (2001).
[13] F. Füchtner, S. Preusche, P. Mäding, J. Zessin and J. Steinbach,
Nuklearmedizin
47
, 116‒119 (2008).
[14] M. S. Berridge, S. M. Apana and J. M. Hersh,
J. Label Compounds Radiopharm.
52
, 543‒548 (2009).
[15] P. J. H. Scott, B. G. Hockley, H. F. Kung, R. Manchanda, W. Zhang and M. R. Kilbourn,
Appl. Radiat. Isot.
67
, 88‒94 (2009).
[16] J. P. de Kleijn, H. J. Meeuwissen and B. van Zanten,
Radiochem. Radioanal. Letters
23
, 139‒143 (1975).