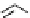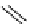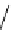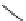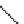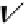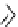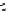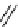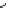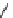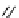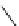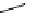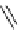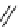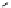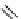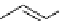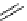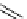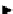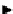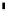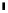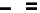Chemistry Reference
In-Depth Information
NH-peptide
NH-peptide
Ar
Ar
H
18
F
N
NN
N
N
N
CH
2
Cl
2
+
+
N
2
RT, 3 min
O
H
93
94
95
Ar
Ar
Peptide
O
18
F
O
18
F
NH
N
Peptide
N
N
CH
3
CN / PBS
N
O
+
O
N
H
N
RT, h
ν
Ph
N
Ph
96
97
98
scheme 3.17
Two prosthetic radiofluorinations, one using the inverse electron demand Diels-Alder cycloaddition of a
trans
-cyclo-octane
(
94
) to an aryl-substituted tetrazine derivative (
93
) and the other the photoactivated addition of a dipolarophilic alkene (
97
) to an aryl substi-
tuted tetrazole (
96
). Ar = Aryl.
(A)
O
O
N
R
2
R
1
O
-N
2
R
1
O
-
NNN
+
H
2
O
+
PPh
2
PPh
2
R
2
90
99
100
OH
O
O
PPh
2
+
R
2
R
1
N
H
101
102
R
2
(B)
O
O
N
-
NNN
-N
2
H
2
O
+
PPh
2
O
PPh
2
R
2
+
O
PPh
2
+
R
1
N
R
2
HS
R
1
S
H
R
1
S
103
90
104
101
105
scheme 3.18
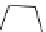
Two variants (A and B) of the Staudinger ligation used in prosthetic radiofluorination. The radioactive part can be either
on R
1
or on R
2
.
The Staudinger ligation [257-260] also employs an organic azide (
90
) that, as in the Huisgen 1,3-cycloaddition, can
either be the derivatised macromolecule or the fluorine-18 bearing counterpart (Scheme 3.18). It reacts with a diphenyl-
arylphosphine (
99
) or a diphenylalkylphosphine (
103
), resulting in an amide bond between the two structures to be linked
(
101
). The reactive groups involved are bio-orthogonal, and the phosphine part is eliminated from the intermediate (
100
,
104
) as a phosphine oxide (
102
,
105
), leaving no trace in the product.
Although thought of as a tool in fluorine-18 bioconjugation, so far only model reactions with relatively small entities have
been presented. Indeed, the method is equally suitable for the construction of small radioligands, such as, for example, the
4-quinolone derivative
109
from precursor
106
(Scheme 3.19) [257]. When the radiolabel is on the azide side as in this
example, 2-[
18
F]fluoroethyl azide (
108
, generated from the tosylate
107
) has been the radiosynthon of choice. With the
Staudinger reaction it gives access to
N
-(2-[
18
F]fluoroethyl)amides, which are difficult to make by a direct SN
2
fluorination
because the corresponding precursors tend to give an intramolecular cyclisation with the amide moiety to 4,5-dihydro-
oxazoles. It provides an alternative to coupling reactions involving 2-[
18
F]fluoroethylamine [261]. The N-H group would
also need protection in a direct fluorination, but not with the Staudinger ligation.
Other model reactions involving 2-[
18
F]fluoroethyl azide (
108
) have also been designed according to variant B in
Scheme 3.18 [259]. Indeed, structure
103
looks more amenable to derivation with complex molecules than
99
. When the