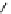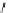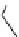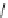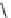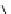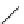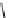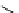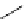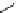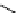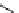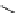Chemistry Reference
In-Depth Information
H
3
C
CH
3
O
O
O
+
NR
3
+
NR
3
18
F
-
18
F
-
O
O
O
O
Cs
+18
F
-
+
N
Resin
K
+
N
N
18
F
-
K
+
O
O
OO
H
3
C
O
CH
3
18
F
-
18
F
-
8
9
10
11
12
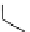
fIgure 3.2
various forms of [
18
[18F]fluoride, activated by dehydration.
radiofluorinated nucleotides and sugars and fluoroacetic acid [27-34, 34, 35]. Besides this special case, there are more
examples where hydration of the fluoride is not prohibitive for nucleophilic substitution. This is when the elements silicon,
boron, or aluminium carry the leaving group [36]. The driving force of these reactions is thought to be the favourable
high strength of the fluorine-silicon, -boron, and -aluminium bonds so that the reaction can take place in an aqueous medium.
In line with this, aluminium species leaching from borosilicate glassware were shown to have a negative impact on [
18
F]
fluoride reactivity [37]. As far as radiofluorination on a carbon centre is concerned, the [
18
F]fluoride normally is rigorously
dried and activated. One method has been to trap it on an anion exchange resin (
8
, Figure 3.2) and use the [
18
F]fluoride-
loaded resin as a reagent [18, 38]. This method is not commonly used today. However, a strong anion exchanger in the form
of a commercially available solid phase extraction (SPE) cartridge is currently used for initial trapping of the [
18
F]fluoride
from the target water. It is then eluted with a mixture of acetonitrile (MeCN) and water containing a suitable base. The eluate
is evaporated to dryness at elevated temperature; often this azeotropic process is repeated several times after adding MeCN.
The base can be, for instance, cesium carbonate or -hydroxide, leaving the fluoride anion beside the relatively large cesium
counter ion in a more or less naked reactive form (
9
). More frequently, instead of the cesium cation, a bulky tetraalkylam-
monium ion, usually tetrabutyl (
10
), is applied by using the corresponding hydroxide or (bi)carbonate as base. Most popular
is potassium carbonate in conjunction with the cage-ligand kryptofix-222 (
12
), the latter encapsulating the potassium cation
and promoting [
18
F]fluoride in its naked reactive form. Sometimes kryptofix-222 is replaced by 18-crown-6 (
11
) in cases
where the substrate is too reactive and would react with the nitrogen atoms of kryptofix-222 [39-42].
However, in many cases drying does not need to be pushed to extremes; small amounts of water are often tolerated and
sometimes deliberately added after drying to diminish loss of [
18
F]fluoride through adsorption to the reaction vessel wall [39,
43, 44]. The tolerance for some water may be useful because it offers a possibility to simplify the drying procedure, which is
much sought after in the application of microfluidics in PET chemistry. This recent development consists of the use of reactor
devices that mix reactants via microchannels and microvessels (down to sub-millimetre scale) in very small amounts allowing
miniaturisation of the process and making it more efficient due to the high surface-to-volume ratio [45-47]. In this context, an
older method [48] of [
18
F]fluoride isolation was recently revived and improved. [
18
F]Fluoride was extracted from the cyclotron
target on a cartridge containing a water-wettable macroporous co-polymer loaded with the long-chain phase-transfer catalyst
N
-tetradecyltrimethylammonium bicarbonate. After purging with a nitrogen gas stream, the radioactivity could be eluted with
1 mL of dry MeCN because the tetraalkylammonium [
18
F]fluoride and subsequent radiochemistry could be performed in this
eluate, which contained about 5000 ppm (~200 µmol) of water. Evaporation to dryness is circumvented because this method
is compatible with closed systems such as microchannel devices [49]. Reversed-phase cartridges have also been used [50]. In
a similar vein [
18
F]fluoride, trapped and 'dried' on the usual anion-exchange SPE cartridge, can, as an alternative to the con-
ventional MeCN/aqueous potassium carbonate mixtures, be eluted with wet MeCN (e.g., 5000 ppm of water) provided with a
strong organic base. The formed hydroxide anions push the [
18
F]fluoride off the exchange sites. Again, nucleophilic substitution
reactions are possible directly in the eluate. Instead of water, traces of alcohols such as methanol can be used [51]. A similar
way of [
18
F]fluoride recovery consists of elution with tetrabutylammonium mesylate or 1-butyl-3-methylimidazolium triflate
in methanol, but in this case the solvent is evaporated before further chemistry [52]. A completely different approach, avoiding
the evaporation step, is the electrochemical isolation of [
18
F]fluoride from an aqueous solution on a positively charged elec-
trode [53] and its subsequent release, after rinsing the electrode with dry solvent, into a solution of kryptofix-222 in MeCN by
reversing the voltage [54, 55]. This procedure has now been miniaturised in a microchannel device for microfluidic radiochem-
istry [56]. Notwithstanding the tolerance of water in a number of nucleophilic radiofluorination reactions, the search for highly
reactive anhydrous [
18
F]fluoride continues. The idea of carrying over [
18
F]fluoride as an intermediate volatile product is well
known. An example is the use of the volatile trimethylsilyl [
18
F]fluoride, which can be easily made from [
18
F]fluoride and
(CH
3
)
3
SiCl in aqueous MeCN and then decomposed again by reaction with tetraethylammonium hydroxide liberating [
18
F]
fluoride [57-60]. A similar approach was recently reported in which conventionally prepared kryptofix-222/K[
18
F]F reacted
with phenyl triflate to give volatile trifluoromethanesulphonyl [
18
F]fluoride. It was carried by a gas stream into a solution of
tributylammonium azide to produce highly reactive tributylammonium [
18
F]fluoride [61].