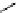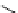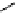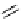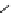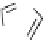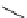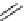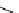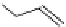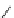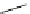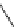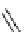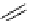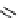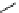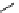Chemistry Reference
In-Depth Information
O
O
N
OH
CH
3
N
HN
HO
O
HO
NH
2
O
CH
3
H
HO
O
N
OH
N
HO
18
F
CO
2
H
O
18
F
CH
3
18
F
O
18
F
1
, [
18
F]Flumazenil
2
, [
18
F]FDG
3
, [
18
F]FLT
4
,
6-[
18
F]uoro-L-DOPA
CH
3
18
F
18
F
18
F
O
N
N
N
CO
2
CH
3
O
N
CH
3
H
3
CO
N
H
H
3
C
CH
3
O
OH
CH
3
N(CH
2
CH
3
)
2
5
, [
18
F]AV-133
6
, [
18
F]LBT-999
7
, [
18
F]DPA-714
fIgure 3.1
Three categories of radiopharmaceutical design: True labelling (
1
), H- or OH mimicking by a [
18
[18F]fluorine atom (
2
‒
4
) and
prosthetic labelling (
5
‒
7
).
and [
18
F]Av-133 (
5
) (vesicular monoamine transporter ligand) [9]. The latter compound shows that an OH for F swap can
change receptor affinity considerably because the corresponding alcohol has a 100 times smaller affinity than [
18
F]Av-133 and
can be tolerated as a contaminant in the radiopharmaceutical preparation [10].
3.2
fluorIne-18: the startIng materIal
Fluorine-18 is conveniently produced with a particle accelerator, normally a cyclotron, by bombarding an appropriate target
with a charged-particle beam. The target can be liquid water or oxygen gas, highly enriched in the isotope
18
O, and the inci-
dent particles are protons with an energy usually between 10 and 20 Mev, inducing the nuclear reaction
18
O(p,n)
18
F [11, 12].
The radioisotope is recovered as an aqueous solution of [
18
F]fluoride. A typical radioactivity level produced is about 1 Ci
having a specific radioactivity (SRA) of 2 to 3 Ci/µmol corresponding to 0.3 to 0.5 µmol of fluoride. Obviously an important
dilution with non-radioactive fluoride occurs because the theoretical SRA of carrier-free fluorine-18 is 1712 Ci/µmol
(0.6 nmol/Ci). A considerable source of carrier fluoride can be radiolysis of commonly used polytetrafluoroethylene (PTFE)
transport lines [13, 14]. SRAs of more than 100 Ci/µmol have also been reported, but it should be noted that these extremely
high values may lead to rapid radiolysis of a radiofluorinated compound [15]. Neutron irradiation of a lithium carbonate
target in a nuclear reactor can be an alternative way of making [
18
F]fluoride [16-18], but this method is seldom used.
Fluorine-18 can also be produced by irradiation of a neon gas target with deuterons by the reaction
20
Ne(d,α)
18
F. This method
is used to make [
18
F]F
2
for electrophilic radiofluorination, for example, the synthesis of 6-[
18
F]fluoro-L-DOPA (
4
), but the
drawback is that the carrier F
2
must be added to extract the radioactivity from the target holder [19]. Electrophilic [
18
F]F
2
is
also made from an [
18
O]O
2
target, equally with added carrier. In this chapter we will not discuss electrophilic radiofluorina-
tion [20, 21] in detail but will focus on the much more current nucleophilic radiochemistry with [
18
F]fluoride [22] illustrated
with recent developments.
3.3
reactIve [
18
f]fluorIde
Radiofluorination with [
18
F]fluoride implies nucleophilic substitution reactions. In these, the [
18
F]fluoride anion attacks the
molecule to be labelled at an atom, normally carbon, that bears a suitable leaving group, which is expulsed while being
replaced by the radioactive fluorine atom. The carbon atom is either aliphatic [23] or aromatic [24-26]. Cyclotron-produced
[
18
F]fluoride comes as an aqueous solution. A fluoride anion in aqueous media is surrounded by a close shell of water
dipoles that effectively hinders nucleophilic action in most cases. For a reaction to take place, the protective water shell must
be broken up, which is not easy where the medium is water. Interestingly, nature has found a way to do this by the enzyme
5′-fluoro-5′-deoxyadenosine synthase isolated from
Streptomyces cattleya
. It was used in
18
F-chemistry to synthesise some