Chemistry Reference
In-Depth Information
O
SO
-
H
Si
O
O
O
N
Na
+
SO
-
N
O
fIGure 16.13
Structure of DY776-silane.
O
O
O
O
124
I Cl
124
I
N
N
O
O
O
O
HO
HO
ScheMe 16.9
The iodination reaction of the Bolton-Hunter reagent.
Cl
-
H
3
N
n
n
SO
3
Na
+
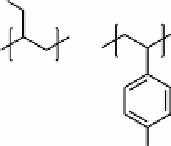
fIGure 16.14
Structures of polyallylamine and polystyrenesulfonate.
difference in the surface charges incurred by the different labels.
Ex vivo
NIRFI of the individual organs shows accumulation
in liver, stomach/intestines, and skin, with minimal intensity observed for kidney, heart, lungs, and spleen [57]. These results
suggest accumulation in the RES organs and that the clearance is achieved effectively by hepatobiliary excretion with no
signs of organ toxicity as determined by Prussian Blue staining [57].
Understanding how nanoparticles cross the blood-brain barrier presents one of the most important challenges to current
biomedical research. AuNPs coated with multilayers of polyelectrolytes have been shown to cross the blood-brain barrier
[59, 60]. However, previous reports indicated that these AuNPs were toxic. In order to reduce toxicity in this study, HSA was
applied as a coating [61].
Polyallylamine and polystyrenesulfonate (Figure 16.14) were used as the multilayers.
In vivo
NIRFI shows that the par-
ticles localise in the head, and X-ray microtomography show boundaries between regions that are not typically obvious in
this imaging technique. The particles were observed in the thalamus and hypothalamus.
Ex vivo
NIRFI shows the particles
in the brain. Confocal microscopy of brain slices indicates that the AuNPs localised in the hippocampus, hypothalamus,
thalamus, and the cortex. FITC on the AuNPs co-localised with Nissl or DAPI stain shows that the particles are in the cells
but not in the nucleus. These areas of the brain are of interest due to their relevance to Parkinson's, Alzheimer's, and prion
diseases.
16.4.2
theranostic applications of nIr-pet bimodal nanoparticle Systems
Polymeric nanoparticles based on the self-assembly of glycol chitosan (Figure 16.15) have been previously reported as drug
delivery vehicles, and N-acetyl histidine can be used to modify the polymer to control the hydrophobicity [62-64]. In this report,
particles of 240-290 nm were prepared using three different weight percentage amounts of NAcHis and labelled with Cy5.5 [65].