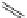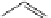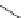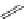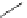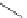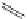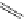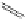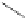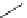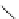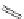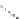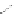Chemistry Reference
In-Depth Information
encapsulated Eu
III
and a maximal two-photon absorption at 700 nm. T24 cancer cells were incubated with the [Eu(L)
3
]
3-
com-
plex and imaged using two-photon excitation at 760 nm (σ
2 PA
(760) = 19 gM). despite the trianionic nature of the complex,
intracellular localisation was observed in the perinuclear region and distributed like ER; bright red spots were also observed
in the nucleus (which could be indicative of nucleoli targeting) [72].
Acyclic derivatives based upon the dTPA ligand core have been utilised for targeting and imaging intracellular zinc
(Figure 12.30). The design rationale for such a species requires a dual functioning binding site with good affinity for Zn
II
and
an ability to sensitise the chelated Eu
III
. The dTPA core was appended with a bridging quinoline-type chromophore, which
was further functionalised with a dipicolylamine unit. The quinoline unit provides a slightly longer wavelength of absorption
in comparison to pyridyl, facilitating excitation at 320 nm. Although the emissivity associated with the free Eu
III
complex
was much less favourable (<1%) than those discussed above, binding Zn
II
induced a favourable 8.2-fold increase in quantum
yield. The use of luminescence lifetime measurements and water relaxivity measurements on the corresponding gd
III
ana-
logue show that the lanthanide coordination sphere is not perturbed by the Zn-binding event. More recent developments have
shown that it is now possible to design Zn-responsive probes perturbing inner sphere Ln
III
hydration and thus induce more
pronounced changes in optical output [73, 74]; similar concepts have now been adopted to target copper [75]. The utility of
the Eu
III
complex to image zinc in a cellular context was demonstrated using HeLa cells. The complex was injected into the
cell, and under ambient levels of zinc, the cells showed no significant emission that could be attributed to the Eu
III
complex.
However, upon addition of a zinc ionophore (pyrithione) and ZnSO
4
(added up to 1 equivalent) the cell image brightened
considerably (Figure 12.30), which was attributed to an increase in emission from the Zn-bound Eu
III
complex. Further, the
effect was reversible: Addition of
N
,
N
,
N
,
N
-tetra(2-picolyl)-ethylene diamine (a membrane permeable chelator) resulted in a
subsequent loss of cellular fluorescence [76].
An emissive Eu
III
complex has also demonstrated promise as a selective probe for imaging singlet oxygen over other ROS.
The complex is based upon an aminocarboxylate-derived 2,2′:6′,2″-terpyridine ligand, which is substituted with a 9-anthryl
unit (Figure 12.31). In its normal form, the complex is weakly luminescent; however, upon exposure to singlet oxygen, the
complex becomes very emissive with long-lived luminescence characteristic of Eu
III
-centred processes. The deactivation of
the non-radiative quenching pathways (i.e., the 'switching on' of the emission) occurs via the anthryl moiety, which reacts
with singlet oxygen, generating an endoperoxide. Methyl-substitution in the 10-position of the anthracene group signifi-
cantly accelerates the rate of this reaction (~
K
10
10
M
-1
s
-1
) and thus increases the sensitivity of the probe. The application to
time-gated luminescence imaging microscopy has been demonstrated using HeLa cells co-incubated with the complex and
ubstituted porphyrin as photosensitser, the latter providing
in cellulo
generation of singlet oxygen upon selective irradiation.
The photosensitiser predominantly localises in the nuclei, and it is these regions that demonstrated the fastest increases in
-
O
O
N
N
O
N
N
N
O
Eu
N
N
O
H
O
O
O
O
FigUre 12.30
Eu
III
complex structure of the zinc-responsive probe.
-
-
O
O
N
N
N
N
1
O
2
N
N
Eu
Eu
O
O
O
N
O
N
N
N
O
O
O
O
O
O
O
O
O
O
O
O
FigUre 12.31
A singlet oxygen reactive Eu
III
complex.