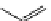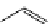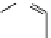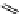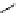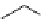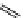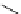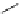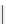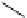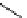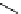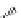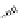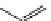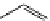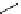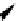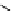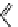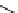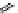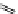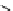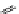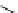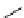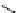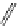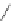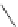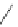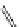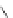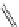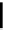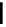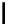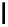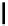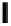Chemistry Reference
In-Depth Information
O
O
O
H H
H
3
C
N
NH
2
O
-
N
OH
O
PO
N
NH
2
Tyrosine
λ
em
= 300 nm (2.5 ns)
O
H
3
C
N
N
O
HO
OH
OH
OH
NH
2
O
N
OH
N
O
HO
O
P
O
-
O
N
N
O
OH
OH
NH
2
OH
OH
Reduced nicotinamide
adenine dinucleotide (NADH)
λ
em
= 450 - 500 nm (0.3 ns)
Phenylalanine
λ
em
= 280 nm (7.5 ns)
Riboavin
λ
em
= 520-570 nm (4.12 ns)
fIgure 11.36
Examples of endogenous fluorophores responsible for
in vitro
/
in vivo
autofluorescence lifetime imaging [159-162].
Br
-
N
+
H
2
N
NH
2
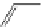
Pyrene
λ
abs
= 450-550 nm (90 ns)
Ethidium bromide
λ
em
= 530-630 nm (20 ns)
fIgure 11.37
Examples of exogenous fluorophores
(
>90 ns emission lifetime) responsible for
in vitro
/
in vivo
fluorescence lifetime
imaging [159-162].
11.6.2
fluorescent lifetime Imaging with exogenous probes
Although autofluorescence lifetime imaging does not require complicated staining procedures, it has a number of limitations
due to its weak and nonspecific fluorescence signals originating from a variety of endogenous fluorophores. Since the emer-
gence of fluorescence lifetime as an imaging modality, many commercially available and specially designed dyes have been
tested for their applications in time-resolved bioimaging. For efficient fluorescence lifetime imaging with exogenous fluo-
rophores, several factors should be considered. These include the use of fluorescent molecular probes (i) with longer life-
times than those of their endogenous counterparts, (ii) in the spectral window with minimal fluorescence from endogenous
fluorophores, and (iii) with dominant fluorescence that overwhelms the contribution of autofluorescence to the measured
lifetime. Figure 11.37 shows some exogenous organic probes of the emission lifetime greater than 90 ns for
in vitro
and
in
vivo
imaging [163, 164].
Pyrenes, polyaromatic compounds with exceptionally long fluorescence lifetimes, have been used for rapid and sensitive
detection of the plated-derived growth factor and mrNA [165], whilst pyrenes with sufficiently long emission lifetimes have
been developed as oxygen sensors. However, as the partial pressure of oxygen (
P
o2
)
in vitro
is in the range of 0.7-2.3 kPa
(depending on organelles, such as mitochondria, which have a
P
o2
of 0.7 to 1.3 kPa), the sensitivity of pyrenes for oxygen
(1 kPa) may not be sufficient to be used as quantitative
in vitro
oxygen sensors [166].
11.7
bIoluMInescence In Molecular IMagIng
Naturally occurring bioluminescence is a process, in line with chemiluminescence, by which living organisms emit visible
light either inside or outside the cell through chemical reactions. It is one of the luminescence processes exhibiting the high-
est quantum efficiency, nearly up to 95% luminescence quantum yield [167]. The basic principle of bioluminescence is that
two types of substances combine together in a light-producing reaction: luciferin and luciferase. Luciferin is a light-producing
pigment that reacts with oxygen to yield oxyluciferins and light, while luciferase is a catalysing enzyme that triggers