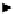Chemistry Reference
In-Depth Information
11.5.3
two-photon Induced
In Vivo
Imaging
With the advantages of the deep tissue penetration by NIr radiation, two-photon fluorescence imaging (especially combined
with fluorescence lifetime imaging to remove interferences from the background autofluorescence of biological specimens)
has contributed significantly to the
ex
(tissues) and
in vivo
(whole body) diagnosis of cancers, ranging from the unveiling of
the basic mechanisms of cancer to clinical diagnostics. These include the search for cancer biomarkers in cells, assessing
histological architecture of the cancer tissue, early cancer diagnosis, and the delineation of the tumour from normal tissues
for staging and surgical removal.
Two-photon imaging of spine dynamics has been carried out on transgenic mice, showing that yellow fluorescent protein
(YFP) or green fluorescent protein (GFP) are overexpressed predominantly in a subset of layer V pyramidal neurons driven
by the Thy-1 promoter [145-153]. Although many mouse lines expressing fluorescent proteins have been generated [154],
some might be too faint for imaging or too dense for the spines to be distinguished from one another. In addition to neuronal
labelling, pretreatments where the skulls of the animals need to be thinned to ~20 μm (known as thinned-skull preparations)
[155] are required before the imaging of fluorescently labelled dendritic spines in the cortex.
11.6
tIMe-resolVed IMagIng
Fluorescence lifetime imaging can be performed either directly by measuring the fluorescence lifetime for each pixel of the
image to generate a lifetime map of the specimen, or via time-gated experiments, where the fluorescence intensity for each
pixel is determined after a short time-lap of photo-excitation to generate an intensity map (Figure 11.35). The former method
is generally used in the monitoring of functional changes caused by environmental factors, while the latter offers the poten-
tial to eliminate background fluorescence and enhance imaging contrast [156].
It should be noted that both linear and two-photon induced fluorescence imaging and time-resolved imaging are also
affected by numerous factors that were mentioned earlier, such as FrET, excimer formation, intersystem crossing, and the
presence of internal quenchers. Compared with lanthanide or transition metal systems, the lifetime of organic fluorophores
is much shorter. This, in fact, makes them more suitable for time-resolved imaging because commercial available FLIM sys-
tems nowadays are only capable of monitoring emission lifetime from nanoseconds to a few microseconds, given that
coordination complexes with very long lifetimes are not suitable for fast scanning devices used in fluorescent lifetime
imaging microscopy [157]. Furthermore, the quantum efficiency of organic fluorophores, which are often >50 %, is much
higher than lanthanide/transition metal systems (~30%) [158].
11.6.1
fluorescent lifetime Imaging with endogenous probes via autofluorescence
Autofluorescence lifetime imaging is an attractive modality because it does not involve
in vitro
staining procedures, and it
purely relies on endogenous fluorophores, such as tyrosine, phenylalanine, riboflavin, and NADH (Figure 11.36). It provides
rich information detailing the morphological and organisational structure of cells and tissues. In general, autofluorescence
lifetime imaging is capable of differentiating one type of tissue from another, healthy from pathologic tissues.
Lamp
pulse
Lamp
pulse
Short
life time
emission
Long
life time
emission
Luminescence imaged
by image camera
T
Time gate
“on”
Time
Time
Time
fIgure 11.35
Illustrations of the time-resolved detection. In the experiment the short-lived fluorescence from the matrix (for endog-
enous probe: eliminate the signal from all parts inside the cell except endogenous probe; for exogenous probe: eliminate the entire
emission signal
in vitro
) has decayed before the gate is turned on and the photoluminescence from the emissive probe is exclusively mon-
itored. This microscope detection enables us to study the single cell.