Chemistry Reference
In-Depth Information
agents are injected in large doses especially compared with the quantities used in nuclear imaging techniques. For
example, Gd-based contrast agents are injected intravenously at a dosage of 0.1 mmol/kg. relaxivity is the ability of a
magnetic contrast agent to increase the relaxation rates of the surrounding water proton spins, which depends on the
molecular structure and kinetics of the complex. The relaxivity (r
1
or r
2
) of a contrast agent in water is the change in 1/T
1
or 1/T
2
of water per concentration of contrast agent and is dependent on parameters such as temperature and magnetic
field. The relationship between T
1
, r
1
, and the concentration of the paramagnetic material is given in Equation (1.4) where
M is a magnetic ion [29].
+
[]
1
/
T
=
1
/
T
rM
(1.4)
(
)
(
)
1
Measured
1
Water
1
Because most T
1
contrast agents are unable to cross the intact blood-brain barrier, they are not used for brain imaging.
Studies have shown that T
2
-weighted imaging is more sensitive for detecting brain pathology as well as for imaging edema.
However, the use of functional MrI (fMrI), a branch of the MrI modality, is more commonly used to measure brain activity
in response to specified stimuli. This technique allows study of the functions of the living brain in a noninvasive manner and
is clinically used in the treatment of brain tumours [29].
1.5.2
Advantages and limitations
MrI provides excellent tissue contrast and higher spatial resolution and is one of the best techniques for showing ana-
tomical detail. It is very useful in early-stage diagnosis of diseases, especially for brain tumours, and in providing
information on the biochemistry and metabolism of tissues. It does not require any ionising radiation, and simulta-
neous extraction of physiologic and anatomic information is possible. Spatial resolution can be increased by increasing
the acquisition time without endangering patients, compared with radioisotope imaging techniques. fMrI allows non-
invasive imaging of the brain without the use of any external contrast agents, but the images are of lower spatial reso-
lution. Advances in technology design allows for a smaller receiver coil radius and high magnetic field strengths to
improve signal to noise ratio and resolution. However, this gives rise to other technical challenges, such as artefacts
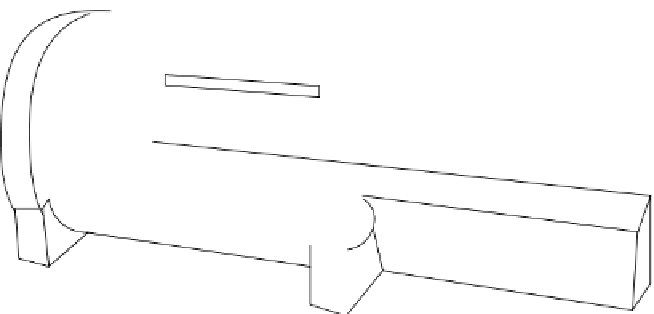
and problems of physiological effects on human patients. Figure 1.17 schematically shows the basics of an MrI
machine.
In terms of disadvantages, MrI is several orders of magnitude less sensitive than nuclear imaging modalities
because only a small percentage of protons are able to absorb the radiofrequency energy to generate a data signal.
reliable signal amplification strategies as well as good contrast agents are thus required. A good contrast agent can
improve the sensitivity of the signals because it alters the relaxation times of tissues in the area but only in regions in
which the contrast agents are concentrated. MrI also requires a much larger dosage of contrast agents than, for
example, radiotracers in nuclear imaging, making toxicity of the imaging agents a major area of concern in molecular
design and development. rigorous tests and clinical trials are thus required in order for MrI contrast agents to be
approved by the FDA.
Main magnet
Shim coils
Gradient coil
RF coil
FIgure 1.17
components of a typical MrI scanner.