Chemistry Reference
In-Depth Information
Chemical
Exchange
RF pulse
Agent labile
protons
Bulk water
protons
Agent labile
protons
Bulk water
protons
Agent labile
protons
Bulk water
protons
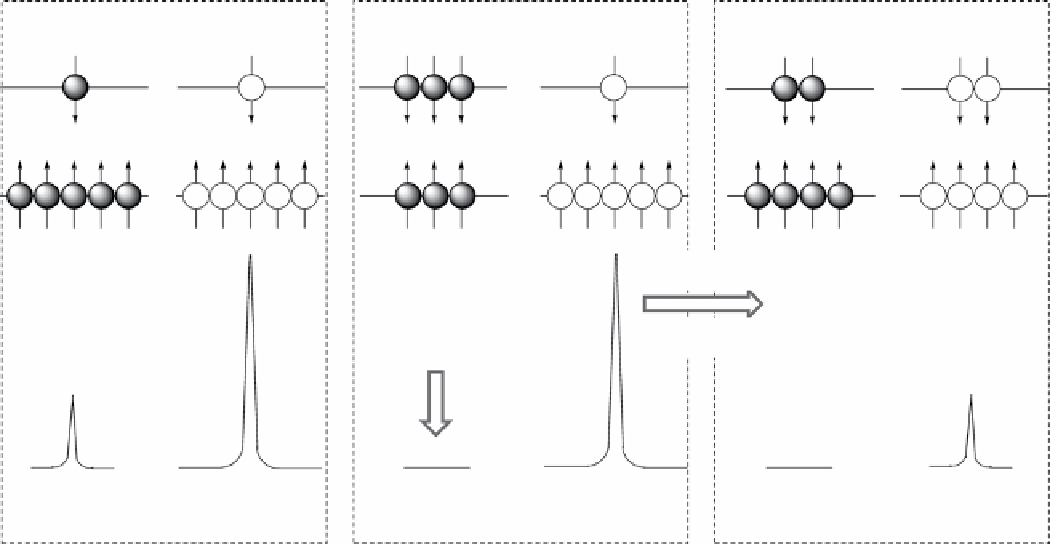
fIgurE 10.1
Illustration of the CEST mechanism showing the Boltzmann distribution of proton spins and the simulated NMR spectra
for two chemically distinct pools of protons. Application of a frequency selective RF pulse causes saturation of the NMR signal, which is
then transferred to the bulk water proton pool by chemical exchange.
Any molecule with labile protons (-NH, -OH) can potentially be used as a CEST agent, provided that the exchange of
these protons with the bulk water protons occurs at a rate (
k
ex
) that is slower than the frequency difference (Δω) between the
exchanging pools (Eq. 10.1).
k
ex
∆ω
(10.1)
Many types of molecules fit this requirement, including hundreds of low-molecular weight compounds containing -NH or
-OH protons [4-7], macromolecules [8-11], and nanoparticles of various types [8-15]. In this chapter, small-molecule
CEST agents will be divided into classifications of diamagnetic (DIACEST) and paramagnetic (PARACEST) agents. Given
that DIACEST agents typically have proton chemical shifts less than 5 ppm away from water, proton exchange must be quite
slow to meet the CEST requirement, whereas the chemical shift of protons in PARACEST agents can vary widely, often
lying more than 100 ppm from bulk water. This means that chemical exchange can be quite fast in PARACEST agents and
still meet the CEST requirement defined by Eq. 10.1.
10.2
dIaMagnEtIc cESt agEntS
Agents of this class are characterised by exchangeable protons from a variety of functional groups such as hydroxyl, amide,
and amine groups that reside within 10 ppm of bulk water protons [16]. Although the small Δω of these agents could prove
disadvantageous due to an increased probability of directly saturating the bulk water protons, if the exchange rates of the
labile protons are slow enough on the NMR timescale, appreciable CEST contrast can be generated using DIACEST agents.
While a number of other functional groups are capable of producing CEST contrast, amide protons represent the most attrac-
tive means of generating CEST because they exchange with water protons more slowly than hydroxyl and amine protons and
the chemical shifts of amide protons are typically further downfield of water than most other types of protons. The abundance
of amide protons in endogenous proteins and peptides has also facilitated the
in vivo
translation of DIACEST MRI through an
imaging technique now referred to as amide proton transfer (APT) [17, 18]. Note that APT and DIACEST are identical
processes but the acronym APT should be used only when the experiment reflects exchange of amide protons specifically.
Amide proton exchange is highly pH dependent. Below ~ pH 5, amide proton exchange is typically too slow for CEST;
above pH 5, base catalysis of amide protons accelerates proton exchange almost linearly with increasing pH [2, 18]. This