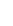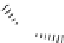Chemistry Reference
In-Depth Information
Protein
O
O
O
C
O
O
O
C
C
H
CH
H
H
H
H
HN
CH
C H
N
CH
C H
C
CH
OH
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
N
N
N
N
N
N
NH
N
N
NH
N
N
O
Ni
O
O
O
N
O
O
R
FIgure 9.11
schematic representation of a histidine tag-nickel nitrilotriacetic acid (NTA) interaction.
strategies detailed in Figure 9.10 to bind a biomolecules to the nanoparticle and then exploit its affinity and specificity
toward the targeting molecule. The most commonly used of these interactions is the one between biotin and avidin/
streptavidin. Biotin can be easily coupled to any other linker/spacer, protein/peptide, or chemical through peptide
chemistry. It is small enough not to substantially modify the properties of proteins when it is attached to them, and
its partner for the interaction is avidin, neutravidin, or streptavidin. All of these proteins present high affinity and
specificity toward biotin, with avidin having a slightly higher affinity (K
d
≈ 10
-15
M). These proteins present several
binding sites in the same molecule to attach biotin (avidin can bind up to four biotin molecules), so they can be used
sandwich-like, first to couple to biotin in the nanoparticle and then to bind a biotin-modified targeting molecule
to the final complex. examples using this methodology include the coupling of antibodies and dyes, for example
[93, 131, 155, 156].
A pair of proteins that have been tested in the functionalisation of MRI nanoprobes are A/G-IgG antibodies. A and
G are small proteins originally isolated from the cell wall of bacteria (they are now available from recombinant sources)
that bind with high affinity and specificity to IgG antibodies (protein A also binds with lower affinity to Ige, M, and A).
As before, the strategy details the use any of the chemical approaches described above, usually peptidic chemistry, to
couple either of the proteins to the nanoparticle and then incubate these nanoparticles with a buffer solution of the
desired antibody[129, 141, 157, 158]. A further strategy involves the use of genetically modified proteins bearing what
is known as the histidine tag (Figure 9.11). This is a sequence of between 5 and 9 histidine amino acids fused usually
at either the N- or the C-terminus of the protein. This motif allows the protein to bind to metal chelates, such as nitrilo-
triacetic acid (NTA) or iminodiacetic acid (IDA).
An advantage of this approach is that there are commercially available antibodies targeted to the histidine tag
sequences that are very convenient to detect. However, the preparation of recombinant proteins with these sequences is
not easy, and this limits the applicability of this approach, but the strategy has been followed to prepare MRI probes
conjugated to peptides and proteins [141, 158, 159].
9.7
applIcatIons
This section discusses some selected applications to illustrate the different fields in which nanoparticles and MRI have been
applied within biomedicine, but does not aim to provide an exhaustive list. Much work with nanoparticles has been carried out
toward the application of MRI not only to diagnose but also to follow the evolution of lesions along the therapeutic process. The
scientific community is currently working hard on the design and preparation of targeted contrast agents, but the examples of
in
vivo
application of these kinds of probes is still scarce. el-Boubbou et al. reported the preparation of magnetic nanoparticles as
MRI probes but not to use within the MRI imaging technique but to obtain information from the T
2
values [108]. They prepared
a series of magnetic nanoparticles functionalised with different carbohydrate moieties to ascertain the differences between healthy
and tumoural cells. Carbohydrate display on cells is only now beginning to be considered as a possible key feature for the diag-
nosis of diseases. To date, carbohydrate biological interactions have been much less explored within biology due to the fact that