Chemistry Reference
In-Depth Information
(a)
(b)
(c)
2
1
3
4
5
6
Single crystalline
Poly crystalline
Gold
100 nm
5 m
Cobalt
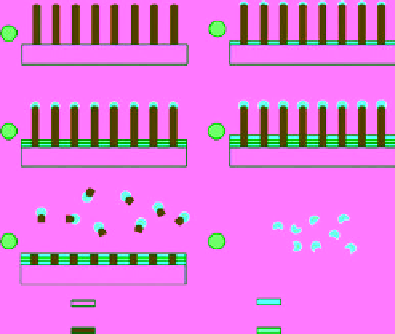
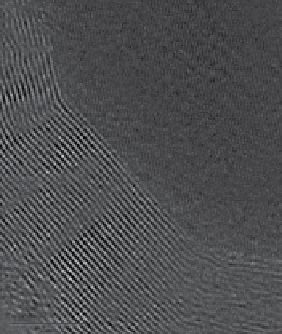
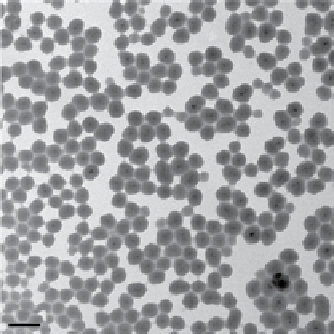
FIgure 9.9
(a) TeM micrograph of siO
2
coated iron oxide nanoparticles. Reproduced with permission from Ref. [106]. (b) Fabrication
procedure of the nanowontons including six steps: (1) etching polysilicon nanopillars on the surface of single crystalline silicon wafer;
(2) deposition of a 10 nm gold thin film; (3) deposition of a 10 nm cobalt thin film; (4) deposition of a 10 nm gold thin film; (5) etching
polysilicon nanopillars in KOH batch solution; and (6) removal of polysilicon by basic etching. Adapted with permission from Ref. [114].
(c) High-resolution transmission electron microscopy (HRTeM) image of graphite encapsulated iron nanoparticle. Reproduced with
permission from Ref. [117].
application within MRI probes [69]. seo and co-workers prepared FeCo nanoparticles and then coated them with a
few layers of graphitic carbon by chemical vapour deposition at 800°C using methane as carbon provider. Finally, to
stabilise these nanoparticles in aqueous conditions, they used modified phospholipids exploiting the interaction bet-
ween the carbon layers and the fatty acid chains of the phospholipids. There are a few papers where this approach
has been used to stabilise other types of nanoparticles, most of them metal-only nanoparticles that are highly sensitive
to air oxidation (Figure 9.9c) [115-118]. To date, though, none of these materials have reached MRI testing.
9.6
FunctIonalIsatIon and surFace ModIFIcatIon
The current state of the art in MRI contrast agent design requires functionalisation of the contrast agent that gives it speci-
ficity. Therefore, the last steps in the preparation of these probes is often the coupling of the targeting moiety. Many molecules
have been used as targeting motifs, with special attention given to natural biomolecules. For the coupling of these biomole-
cules to the nanoparticles, it is highly desirable to use rapid, high-yielding reactions carried out under mild conditions in, as
far as possible, aqueous media. An extensive description of conjugation techniques is featured in Chapter 2, thus only the
most common functionalisation methodologies followed in nanoparticle work will be mentioned here (Figure 9.10). The
simplest strategy is to just incubate a solution of the nanoparticles with a solution of the targeting ligand, where the high
surface to volume ratio of the nanoparticles helps in the adsorption of the biomolecules. The biomolecule-functionalised
nanoparticle is then stabilised either by electrostatic forces or, in most cases by weaker van der Waals interactions or hydrogen
bonds [119-121]. For example, the amount of proteins that can be bound in this way to the nanoparticles is not negligible
[119]. Both DNA and RNA molecules are biopolymers that present a global negative charge under physiological conditions,
thus they can be electrostatically attached to positively charged nanoparticles (usually functionalised with terminal amine
groups). Here there are issues with the stability of the resulting nanoparticles, that is, pH, ionic strength, or concentration
variations can desorb the bound biomolecules. This is the main reason why more complex covalent approaches are generally
preferred nowadays.
• Peptidic bonds: The coupling of an amine group to a carboxylic acid to form an amide is a versatile reaction that can be
carried out in aqueous buffers under mild conditions. Most peptide sequences and proteins contain both primary amines
and terminal carboxylic acids. Other common nanoparticle ligands also present these kind of groups (e.g., citric acid,
APTes) or they can be chemically modified to present them (e.g., dextran can be easily transformed into carboxy-
dextran or amino-dextran). An advantage for
in vivo
work is that the new bond is already present in the organism thus no
adverse effects are expected. The coupling reaction requires activation of the carboxylic group and eDC, 1-ethyl-3-(3-
dimethylaminopropyl)carbodiimide hydrochloride), sometimes combined with NHs or sulfo-NHs (N-hydroxysuccinimide,