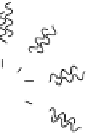Chemistry Reference
In-Depth Information
X
X
X
X
Ligand exchange
Inorganic coating
Organic coating
Y
Z
Z
Y
Y
Y
X
Z
X
Y
Y
Y
X
X
Z
Y
FIgure 9.7
schematic representation of the different strategies available to form water soluble and functionalisable nanoparticles. The
final ligands on the nanoparticles can be either individual molecules or polymers.
9.5.1
ligand exchange
In this process, the ligands on the nanoparticles are replaced by more suitable ones, usually bifunctional species. This can be
performed in one step, just mixing the nanoparticles with a large excess of the new ligands, or in two steps, stripping first the
nanoparticles and then introducing the new ligand. With metal oxide nanoparticles, molecules bearing carboxylic acids are
the preferred ligands [25, 50, 54], but alternatives include diols (usually dopamine-based molecules) [74-77], molecules
with phosphate/bisphosphonates groups [78, 79], or hydroxamic acids [80]. Phosphonate groups have been introduced more
recently than carboxylic acids, and their bonds to oxide nanoparticles are more stable even at high temperatures [81]. The
way in which the ligands interact with the metallic atoms on the surface of the nanoparticles depends on (i) the chemical
nature of the ligand itself, (ii) their ability to pack and form self-assembled monolayers (sAMs) on the surface of the
nanoparticles, (iii) the nature of the nanoparticle, and (iv) the curvature of the nanoparticle's surface. For the case of
carboxylic ligands, three different interaction modes have been proposed [82]. In the monodentate situation, only one oxygen
atom binds to the surface. When both oxygen atoms are involved in the bonding, they can either be attached to the same
metallic ion (chelating situation) or to different atoms (bridged situation). A similar situation has been proposed for phos-
phates; one or two oxygen atoms from the phosphate group can interact with Fe
3+
ions on the surface of the nanoparticle
[83]. The case of FePt nanoparticles is different because the presence of platinum atoms on the surface of the nanoparticle
allows the use of thiol-noble metal chemistry for the ligand exchange [28, 29]. This chemistry has been widely studied for
the functionalisation of gold nanoparticles [84] and the sulphur-metal bond is more stable than the ones described above,
making the ligand exchange process easier. In general, this strategy can provide individual and monodisperse nanoparticles
without a dramatic increase in the size of the particles, but due to the nature of the bond between the ligands and the inor-
ganic core, some stability issues can arise.
A similar approach toward the preparation of water soluble nanoparticles is the coating of the nanoparticles with a
polymer. In this case, the stability of the resulting nanoparticles is generally higher, but the hydrodynamic size of the
nanoparticles is drastically increased, and samples in which several nanoparticles are encapsulated inside the same polymer
chain are often found. Bifunctional or natural biocompatible and biodegradable polymers can be used; among the natural
polymers (usually carbohydrate-based materials) dextran [17] is the most preferred. Dextran is a non-charged polymer of
D-glucose that can be easily modified (even once on the nanoparticles [85]) to present carboxylic or amino groups on its
surface. As mentioned in previous sections, dextran can be introduced directly into the nanoparticle formation reaction, or it
can be used after their formation to provide water solubility and biocompatibility. When considering synthetic polymers,
many different options have been explored (Figure 9.8), from polystyrene sulfonate (Pss) [55], to the biodegradable
poly(lactic-
co
-glycolic acid) (PLGA) [86], to polyethyleneimine (PeI) [87], to more complex approaches such as layer-by-
layer protocols [88]. However, by some way the most used synthetic polymer is polyethylene glycol (PeG) due to its out-
standing properties for biological applications. PeG is a linear, biocompatible polymer whose length can be tailored to suit
particular applications. It has been approved for human applications in pharmacology [89] and has been shown to minimise
unspecific interactions, helping these nanoparticles to evade the mononuclear phagocyte system (MPs) [90]. It is not pos-
sible to use simple PeG molecules for a ligand exchange reaction because the bond between the hydroxyl terminal group of
the PeG and the nanoparticle is not strong enough, but PeG molecules can be chemically functionalised to present different