Chemistry Reference
In-Depth Information
(a)
Gd
2
O(CO
3
)
2
T1 shell (1.5 nm)
SiO
2
separating
layer
MnFe
2
O
4
T
2
core (15 nm)
20 nm
(b)
SiO
2
thickness
4 nm
8 nm
12 nm
16 nm
20 nm
50 nm
50 nm
50 nm
50 nm
50 nm
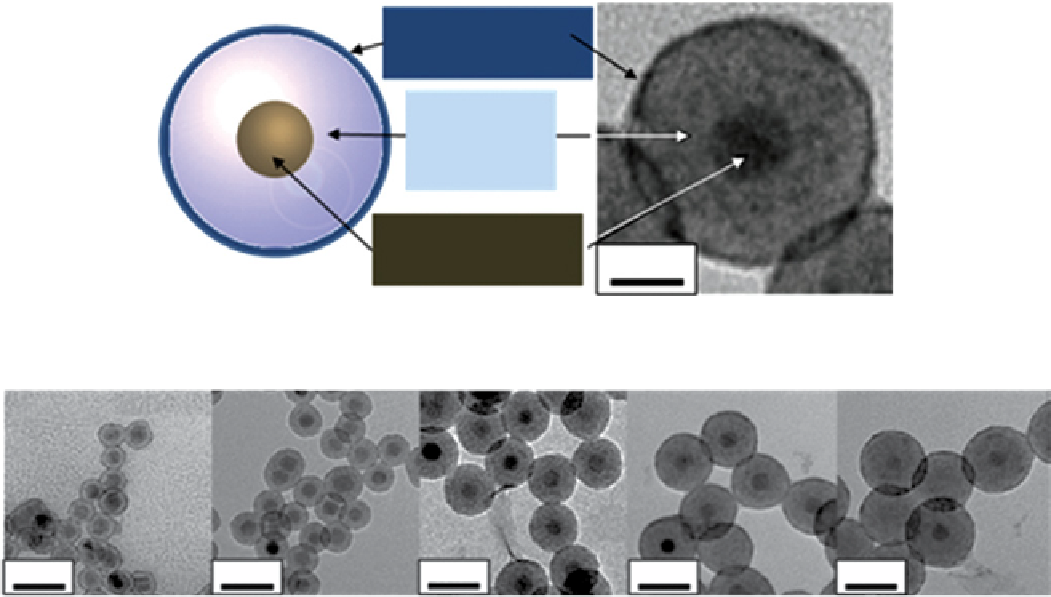
FIgure 9.6
(a) schematic and transmission electron microscope (TeM) image of core-shell type MnFe
2
O
4
@siO
2
@Gd
2
O(CO
3
)
2
nanoparticles. (b) TeM images of the same nanoparticles with variable separating layer thickness (4, 8, 12, 16, and 20 nm), having a fixed
MnFe
2
O
4
core (15 nm in diameter) and a Gd
2
O(CO
3
)
2
shell (1.5 nm). Reproduced with permission from Ref. [70].
Gd by means of the number of DTPA molecules attached per nanoparticle. In a final example, Huang and co-workers
followed a similar strategy except that gadolinium chelates were coupled to the channels of a mesoporous silica shell
[72]. 22 nm Fe
3
O
4
nanoparticles were prepared from Fe(acac)
3,
oleic acid, and trioctylamine, following a thermal decom-
position route. The hydrophobic ligands of these nanoparticles were exchanged for more hydrophilic ones (CTAB), and
the nanoparticles were encapsulated inside mesoporous silica shells in basic water solution using TeOs as the silicon
source. After purification, the particles were heated to reflux in ethanol, and APTes was added to yield amine function-
alised nanoparticles. DOTA-NHs was subsequently coupled to the amine groups, and the resulting product was incubated
with GdCl
3
to yield the final dual MRI probe. In this case, the effect of the T
1
probe over the T
2
one or vice versa was not
further investigated.
9.5
Water soluBIlIsatIon
There are now a number of reliable and well-characterised protocols for the preparation of magnetic nanoparticles to suit
their application or design. The real challenge comes in the functionalisation of the nanoparticles to achieve water soluble,
biocompatible, and specific probes. For years the
in vivo
performance of nanoparticulate MRI contrast agents relied on an
intrinsic difference in uptake between different organs or tissues. This is the case for iron oxide nanoparticle-based contrast
agents currently approved in the clinic. The nanoparticles are cleared away from the blood stream by the liver, thus they are
used for the diagnosis of hepatic diseases. This trend has been moving progressively toward a specific labelling design using
targeting molecules [73], although the strategy has not, as yet, provided good enough results to be applied in the clinic.
As mentioned previously, some of the preparation protocols, for example, co-precipitation ones, are flexible enough to
allow the
in situ
introduction of different ligands. In these cases, water-soluble nanoparticles ready for further functionalisa-
tion can be obtained in one reaction. However, most preparations do not give the option of ligand selection because mole-
cules with highly specific properties are required. In these other cases, the first mandatory step toward the specific
functionalisation of the nanoparticles is the replacement or modification of the ligands (Figure 9.7). There have been a
number of different strategies proposed [34], with the most popular one being ligand exchange.