Chemistry Reference
In-Depth Information
The use of gold nanoparticles has also been widely explored as a 'beacon' to which chelates can be attached. Gold
nanoparticles are easily produced, and well-known thiol-gold chemistry can be explored for the stable functionalisation of
the nanoparticles in a versatile and efficient way. Alric et al. published the
in situ
preparation of 2.4 nm gold nanoparticles
protected with a chelate, DTDTPA, derived from diethylenetriaminepentacetic acid bis-anhydride [60]. Their synthesis was
carried out in a mixture of water, ethanol, and acetic acid using HAuCl
4
as the gold source and sodium borohydride (NaBH
4
)
as the reducing agent. In a second step, the nanoparticles plus the chelate were incubated in the presence of gadolinium chlo-
ride to force the paramagnetic ion into the complex. A very similar strategy was followed by Mayer and co-workers to obtain
T
1
gold nanoparticles [61]. They prepared 3.5 nm gold nanoparticles
in situ
with a thiol derivative of DTTA chelate, and in
a second step incubated them with GdCl
3
. A similar strategy was followed by Marradi and co-workers [62]. In this case they
also used an
in situ
reaction to produce gold nanoparticles functionalised via thiol chemistry with gadolinium chelates
(DO3A type) and different carbohydrates. They showed that it is possible to alter the performance of the nanoparticles as T
1
contrast agents by not only modifying the number and type of chelates, but also by changing the nature of the surrounding
molecules. In this case, the organisation of water molecules around carbohydrates is configuration dependent; this can either
bring water protons closer to the paramagnetic metal centre or keep water molecules apart. using a different strategy, song
et al. prepared 13 and 30 nm gold nanoparticles with gadolinium chelates [63]. First, gold nanoparticles were prepared, and
in a second step a ligand exchange reaction was carried out. The new ligands were thiol-ended modified DNA chains bearing
gadolinium chelates (Gd595) (Figure 9.4c). The ligand exchange with the DNA derivatives was performed over 24 hours,
gradually increasing the concentration of NaCl in the reaction medium (10 mM phosphate buffer saline) up to 0.1 M. In this
case the modified DNA ligands provide not only the T
1
contrast enhancement, but also increase cell permeation and can
serve as cell tracking ligands.
Finally, carbon nanotubes (CNTs) have been used as 'inert' nanoparticulate platforms to couple T
1
chelates. Richard
et al. functionalised the outer surface of multi-walled carbon nanotubes (MWNT) with modified DTPA chelates [64].
They designed the modified DTPA chelate in such a way that it presents an aliphatic chain capable of surface adsorp-
tion on the nanotubes via van der Waals interactions, while the rest of the molecule is a polar head group that confers
water solubility and at the same time is capable of chelating Gd
3+
ions. To functionalise MWNTs with the amphiphilic
ligands, solutions of the DTPA derivative at a concentration above the critical micelle concentration were sonicated
with powders of the nanotubes. A completely different approach was chosen by Tran et al. In their design, paramag-
netic ions are located only inside the nanotubes clustered around defect sites along the sidewalls (Figure 9.5d) [65].
They used single-walled nanotubes produced by an electric-arc discharge method that were then cut into ultra-short
sWNTs (20-80 nm in length) by fluorination and pyrolysis at 1000°C under inert atmosphere. The nanotubes were
reduced with sodium to produce individualised or small bunches of nanotubes and then sonicated in a water solution
of gadolinium chloride. Finally, to solubilise the product in aqueous media, the nanotubes were sonicated with a sur-
factant (Pluronic F-108).
(a)
(b)
O
C
(OCH
2
CH
2
)
45
O
-
P
O
O
O
O
O
NH
2
N
H
O
O
FeCo
20 nm
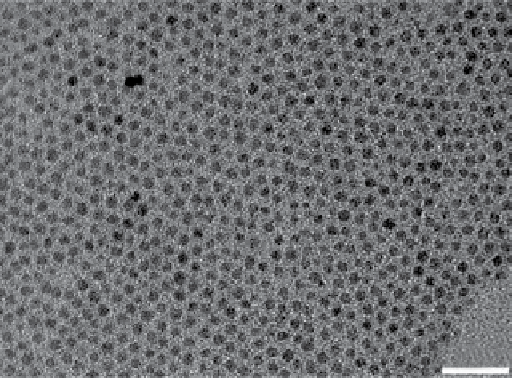
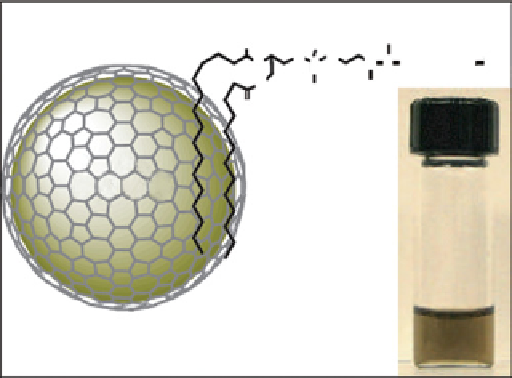
FIgure 9.5
(a) TeM micrograph of 4 nm Fe
3
O
4
nanoparticles. From Ref. [67]. (b) schematic diagram of a FeCo/GC nanocrystal, struc-
ture of the phospholipid molecule used for functionalisation and a photograph of a PBs suspension of functionalised FeCo nanocrystals
taken after heating to 80°C for 1 hour. Reproduced with permission from Ref. [69].