Chemistry Reference
In-Depth Information
(b)
(c)
2000
(a)
(211)
(314)
(224)
(105)
(220)
(211)
(103)
(112)
1500
(103)
1000
(200)
(101)
(224)
(112)
(004)
(321)
(314)
(220)
500
(105)
(312)
30 nm
0
20
30
40
2θ (°)
50
60
(d)
pH4.6
HMON
MON
WMON
(e)
(f)
100 nm
100 nm
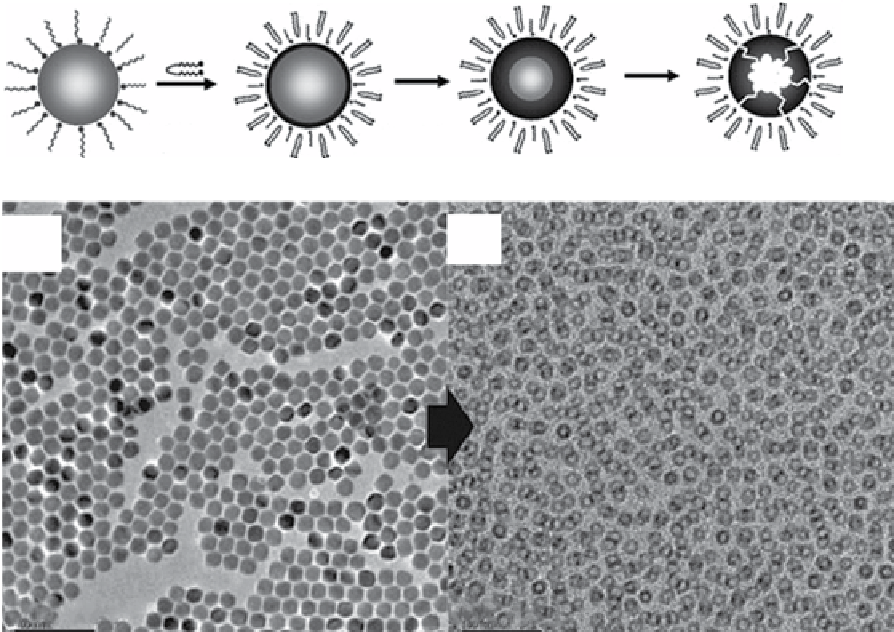
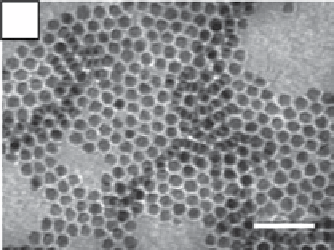
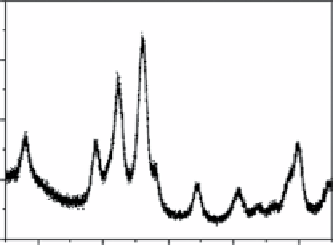
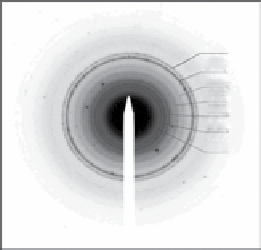
FIgure 9.3
(a) Transmission electron micrograph. (b) X-ray powder diffraction pattern. (c) Indexed selected area electron diffraction
pattern (negative shown for clarity) of 7 nm diameter Mn
3
O
4
nanocrystals. Reproduced with permission from Ref. [30]. (d) scheme of the
formation of Mn
3
O
4
hollow nanoparticles. Reproduced with permission from Ref. [168]
e
and
f:
TeM images showing the changes from
solid nanocrystals of 18 nm-sized MnO (e) to the corresponding hollow oxide nanoparticles (f) through the etching process. Adapted with
permission from Ref. [56].
9.3.3
paramagnetic additions to nanoparticles
A different approach toward the production of nanoparticulate T
1
agents is an evolution of the classic chelates strategy.
Basically, DOTA (1,4,7,10-tetraazacyclododecane tetraacetic acid) or related chelates are functionalised with a linker
featuring a functional group that allows its conjugation to a nanoparticle. In this case, the nanoparticle acts only as a
platform to bind the paramagnetic agents. In this way some solubility issues can be overcome, the local concentration
of the contrast agent is increased, and more importantly, the chelates can be combined with other ligands to provide
other properties (as for example, cell-penetrating abilities). This strategy has been useful for the design of multifunc-
tional nanoparticles, because the nanoparticles themselves can be active in another imaging field (e.g., quantum dots for