Chemistry Reference
In-Depth Information
(c)
(a)
20 nm
(b)
0.2 µm
20 nm
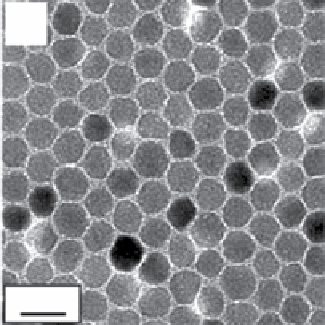
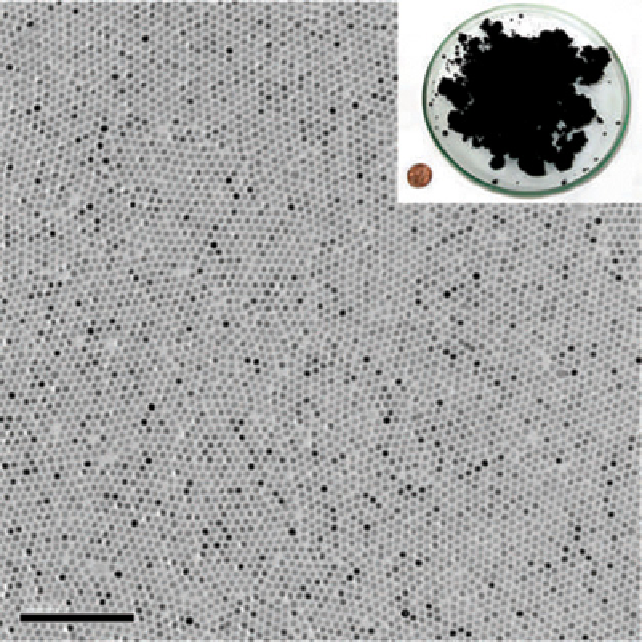
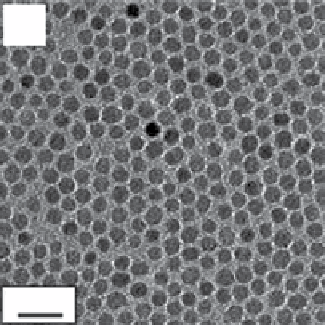
FIgure 9.2
TeM bright field images of (a) 6 nm and (b) 12 nm Fe
3
O
4
nanoparticles deposited from their hexane dispersion on an
amorphous carbon-coated copper grid and dried at room temperature. Reproduced with permission from [24]. (c) TeM overview micro-
graph of 12 nm Fe
3
O
4
nanoparticles. Inset, photograph showing a Petri dish containing 40 g of the monodisperse magnetite nanocrystals
and a u.s. one-cent coin for comparison. Reproduced with permission from Ref. [23].
9.2.1.3 Other Methods of Synthesis
The methodologies described so far are the most popular ones for the preparation of
magnetic nanoparticles for imaging applications, but a number of other chemical approaches have also been described.
• Microemulsion protocols: This kind of protocol can be considered to be an evolution of the classic co-precipitation
methodology. The weak point of the iron salts co-precipitation method is the broad size distribution of the obtained
particles, thus numerous modifications of this synthesis have been described in which the reaction takes place in
constrained environments. surfactant molecules spontaneously form nanodroplets of different sizes when in the
correct solvents and concentrations. Inside these droplets iron salts can be encapsulated in water solutions isolated
from outer organic non-polar solvents. In the case of these reverse microemulsions, the size of the droplets or
micelles can be tuned (by varying the molar ratio of water to surfactant) to be usually between 1 and 50 nm [3]. Two
such microemulsions can be mixed together; upon stirring, the micelles will collide, coalesce, and break up again,
thus mixing their contents and starting the chemical reaction under constrained conditions, thus working as a nano-
reactor [34]. Finally, in the case of nanoparticles, the final size and homogeneity of the population of nanocrystals
will depend on the size and quality of the starting microemulsion. The reactions that can be carried out inside these
systems are not restricted to co-precipitation of iron salts. Indeed, the first example of magnetic nanoparticles pro-
duced using microemulsion approaches was based on the oxidation of Fe
2+
salts into Fe
2
O
3
and Fe
3
O
4
[35]. Depending
on the chemical nature of the reagents needed for the reaction, different types of surfactants can be used, both ionic
and non-ionic ones. some common examples are sodium bis(2-ethylhexyl sulfosuccinate) (AOT) and cetyltrimeth-
ylammonium bromide (CTAB) [36] as ionic ones, whilst Triton X-100, Igepal CO-520, and Brij-97 [37, 38] are
some common examples of non-ionic surfactants.