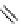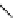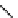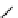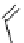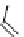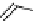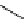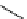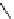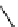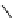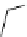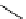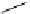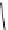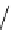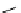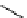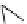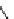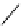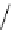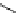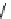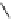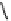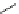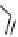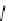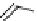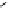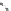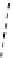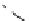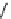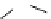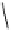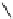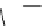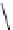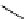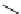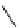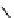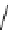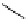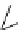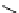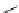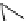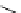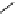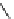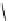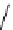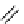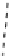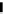Chemistry Reference
In-Depth Information
X
O
X
O
O
O
O
O
O
H
H
X
H
O
OR
O
O
OR
H
H
O
O
O
O
H
OR
OR
H
H
O
X
O
OR
O
H
RO
O
H
O
NH
O
O
O
H
O
O
N
H
O
X
H
H
O
N
Gd
H
O
O
O
N
O
O
N
OR
O
O
N
O
X
O
O
O
O
OR
X
O
OR
O
N
N
RO
O
X=
N
O
NH
N
RO
N
N
O
RO
Gd
R=glucose
O
NN
O
O
O
[Gd.DENgDOTA]
-
Heptagadodextrin
O
O
O
O
O
O
O
O
O
O
N
O
O
O
O
N
N
O
O
O
O
Gd
O
NN
O
O
O
O
O
O
O
O
O
O
O
O
O
N
N
O
O
O
Gd
O
O
O
O
NN
O
O
O
O
O
O
O
O
O
O
O
[Gd.BnODOTA]
-
Gd.DOTACd
fIgure 8.6
Structures of assemblies investigated with respect to increasing relaxivity through increasing τ
R
or the number of gd per
complex.
large relaxivities: It is worth noting that this system has a molecular weight in the region of 5500, and a 'relaxivity per mole
of complex' of around 85 mmol
-1
s
-1
, compared with a molecular weight of around 3200 for the mono-metallic gd.
dENgadOTA complex discussed above. In applications where molecular size and the consequent slow diffusion through
biological media is not an issue, there is clearly much to be said in favour of the approach of using many metal centres.
Parker and co-workers have exploited cyclodextrins in a different way, assembling a multimetallic complex through the
interaction between a dOTA-monoamide derivative (gd.dOTACd) appended with a permethylated cyclodextrin, and
gd.BnOdOTA (Figure 8.6) [79]. They showed that the resulting assembly formed with an association constant at 298 K of
around 200 M
-1
; this means that a mixture of species will exist in solution at the mM concentrations associated with MRI
measurements, and in this case only very small variations were observed between sum of the relaxivities of the individual
components and the total relaxivity of the mixture. By contrast, the same cyclodextrin interacts strongly with MS-325,