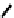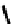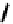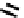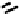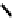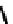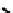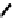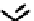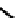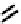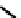Chemistry Reference
In-Depth Information
A variation on the amineoxime ligand theme was the synthesis of complex
76
with the butylene backbone, named
TcHl-91m. This showed promising hypoxia selective uptake but remarkably this was lower than complex
77
(Figure 6.28),
TcHl-91, or Prognox
Tm
, which was originally selected as a non-hypoxic selective control. When the reaction of the amine-
oxime with [
99m
Tco
4
]
−
is carried out in non-aqueous solvents, a mono-oxo complex is formed, but this is rapidly converted
in aqueous media to the
trans
dioxo complex. Cellular uptake studies showed that Tc Hl-91 retention was significantly
higher under hypoxic conditions and that it also has higher uptake in a live isolated ischemic rat heart. In mice CanT tumour
retention was comparable with that of a
123
I-labelled nitroimidazole IAZA, and the uptake was normalised to that of tritiated
mISo [261, 262]. Thus, the hypoxic selectivity of Hl-91 is unambiguous and has been confirmed by subsequent pre-clinical
and clinical investigations [263-269], including correlation with FDG [270], but the mechanism of selectivity remains
unknown. Autoradiography studies show that it accumulates in the cell membrane under hypoxic conditions, but it is not
clear if any bioreductive process is occurring or if this is a response to hif (hypoxia inducible factor) initiated changes in cell
biochemistry. 2-, 4-, and 5-nitroimidazoles [271], misonidazole [272], and nitrophenyl derivatives [273] have been coupled
to the
99m
Tc tricarbonyl core (
78
and
79
), and preliminary biodistributions in tumour-bearing mice carried out. Although
tumour retention was observed and found to be highest for the 2-nitroimidazole derivatives, this has not yet been correlated
with hypoxia by IHC.
There have also been investigations of the effect of coupling more than one nim molecule to a
99m
Tc chelator; examples
appear in
80
and
81
(Figure 6.29). For the propylene amine complex
80
, the introduction of a second 2-nim group raised the
retention of
99m
Tc in murine sarcoma cells under hypoxic conditions from approximately 25% for BmS 181321 (discussed
above) to approximately 60%, suggesting that hypoxic selectivity is increased by adding a second 2-nim group [274].
However,
in vivo
studies have not been carried out. An alternative strategy involved the coupling of four nitroimidazole-
based groups to a
99m
Tc cyclam complex as shown in
81
. Presumably this was the
trans
dioxo derivative, although this was
not explicitly confirmed. For the
99m
Tc derivative of cyclam with misonidazole groups attached, the uptake under hypoxia in
DU-145 prostate tumour cells was only marginally higher than under air, and the
in vivo
tumour uptake was similarly low
[275]. A cyclam
99m
Tc derivative analogous to
81
but bearing four molecules of the hypoxic selective nitrotriazole
82
(AK
2123) has also been reported (Figure 6.30) [276]. Here uptake in rat mammary tumours was correlated with both oxygen
electrode measurements of po
2
and uptake of a
125
I-labelled nitroimidazole derivative, suggesting that this compound was
promising for the clinical imaging of hypoxia, although other cancer types need to be studied. There has also been an
-
O
NO
2
O
N
O
N
OC
OC
N
N
N
Tc
N
N
N
N
N
OC
OC
M
N
M
OC
OC
N
N
NO
2
NO
2
NO
2
O
O
O
O
M=Tc, Re
H
O
O
79
80
81
fIgure 6.29
Further nitroimidazole and nitroaromatic conjugates.
+
R
R
O
N
N
O
Tc
NO
2
N
+
N
O
N
R
R
N
N
O
OMe
R=
N
N
N
N
OH
NO
2
NO
2
81
82
fIgure 6.30
nitroimidazole and nitrotriazole conjugates with Tc cyclam complexes.