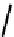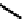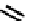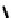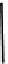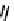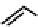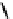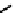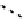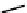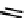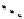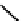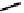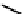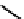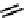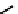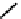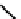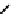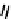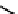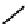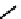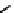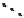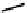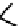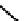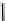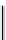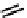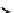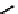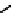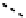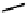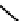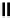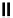Chemistry Reference
In-Depth Information
6.6
ImagIng of hypoxIa wIth
99m
tc
The rapid growth of some tumours is associated with reduced blood supply, particularly toward the centre and the creation of
hypoxic zones. A high proportion of hypoxic tissue generally indicates poor prognosis for the patient partly due to the prob-
lems of delivering effective radiotherapy at low oxygen concentrations [254] and also to the release by hypoxic cells of acti-
vators that can
inter alia
stimulate blood vessel and tumour growth. oxygen concentrations can be determined in tumours by
use of oxygen electrodes, but this method is invasive and can be unreliable [255], particularly with smaller tumours. Hypoxia
is also important in other diseases such as diabetes, arthritis, and heart ischemia. The molecular imaging of hypoxia [256]
therefore has vital role both in determining the optimal course of treatment for a cancer patient [257] and generally distin-
guishing poorly perfused tissue from healthy or necrotic tissue. 2-nitroimidazoles (nims) accumulate in hypoxic tissue via
a redox trapping mechanism, and
18
F-labelled derivatives such as F-mISo
71
are used in the clinic for assessing hypoxia
(Figure 6.27). The copper bis(thiosemicarbazone) complex
64
CuATSm
72
is also in use but operates via a very different mech-
anism from F-mISo. none of the agents in current use have optimal imaging characteristics and involve the use of expensive
PET radioisotopes, thus alternatives using technetium-99m are a worthwhile goal. The confirmation of the hypoxic selectivity
for a new agent is not straightforward. The extent of hypoxia and the time dependence of specific hypoxia uptake
in vivo
are
highly dependent on the cancer type. It is therefore necessary to show that there is hypoxic selectivity in tumour cells
in vitro,
then demonstrate that any tumour uptake
in vivo
is indeed in hypoxic tissue and not merely non-specific by carrying out
immunohistochemical (IHC) staining of tumour sections or at least correlation with oxygen electrode measurements.
The first approaches to technetium agents involved the conjugation of nims to well established chelators such as the
BATo system via the capping boron (
73
). However, enzymatic reduction experiments suggested that rate of reduction was
too low for imaging use [194]. Several nim conjugated derivatives of propyleneamineoxime
99m
Tc complexes have been
explored as potential hypoxic selective agents that differ in the location of the nim group. Complex
74
designated BmS181321
showed hypoxic selective uptake in cancer cells [258] but was later shown to decompose in saline and have non-optimal
biodistribution characteristics (Figure 6.28) [259]. The modification of the propylene backbone and attachment of nim in
75
(designated BRU59-21) improved the biodistribution with rapid blood clearance and improved tumour uptake, which was
shown in phase 1 clinical trials to correlate with pimonidazole hypoxia staining [260].
NO
2
N
N
B
O
O
O
N
N
N
Tc
NO
2
N
N
N
N
N
N
X
N
F
N
N
Cu
O
O
OH
H
H
O
S
S
NHMe
MeHN
71
72
73
fIgure 6.27
FmISo, CuATSm, and a nitroimidazole conjugate.
NN
NO
2
O
O
O
O
N
N
N
N
N
N
N
N
N
N
Tc
Tc
Tc
Tc
N
N
NO
2
N
O
N
O
N
O
NO
2
N
O
N
N
N
N
O
O
O
O
H
H
H
H
74
75
76
77
fIgure 6.28
nitroimidazole conjugates of Tc amineoxime complexes.