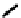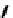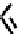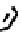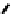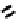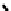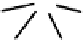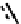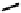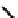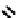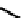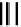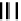Chemistry Reference
In-Depth Information
possibilities are being explored. Thus the complex [
99m
Tco
3
(tacn)] (tacn = triazacyclonononane) has been prepared in moderate
yield from pertechnetate in the presence of a phosphane attached to a solid bead. The earlier reported olefin reaction with a
dioxo unit to give a diol was then exploited to attach potential targeting groups - two examples are
32
(acetylated glucose) and
33
(2-nitroimidazole) [109-111]. The biological properties of these molecules have yet to be reported.
6.2.3
nitrido complexes
The strong π-donating characteristics of the oxo-group makes a major contribution to the stability of high oxidation state Tc
and Re oxo compounds. The trianionic nitride ligand acts in a similar manner, but the additional negative charge opens up a
series of complexes with different co-ligands or overall charges to those with oxo-ligands. The first synthesis of a simple
nitride of Tc or Re was carried out nearly 50 years ago by Chatt et al. who showed that [RenCl
2
(PPh
3
)
2
] and [RenCl
2
(Pme
2
Ph)
3
]
could be made using hydrazine or azide as the source of nitride [112, 113]. This work was subsequently extended to
99
Tc and
99m
Tc through the work of Baldas and co-workers, who reported extensively on nitride complexes and proposed that the
nitride core could be used in
99m
Tc-based radiopharmaceuticals [114-120]. They established that the paramagnetic Tc(VI)
species [TcnCl
4
]
−
could be made by the reaction of pertechnetate with azide in acid solution and investigated its substitution
and redox chemistry. It was not until 1998 that the same synthetic route was successfully applied for the synthesis of
[RenCl
4
]
−
[121]. The coordination chemistries and spectroscopic properties of the Tcn and Ren cores were then explored
further in detail by Abram and co-workers, and some representative examples of this work are shown below (
34, 35, 36
);
these and others appear in the following references (Figure 6.11) [122-126].
The nitride complex baton was then taken up by Duatti and co-workers who showed that somewhat surprisingly neutral
dithiocarbamato Tc nitrides showed promise as heart imaging agents. Detailed optimisation studies led to the TcnoET
compound
37,
which has had clinical trials (Figure 6.12) [127-129].
The mechanism by which it is retained in myocardial tissue is still not clear. A crucial issue was to find a high yield route
to the Re and Tc nitride core that did not require the use of azide. The hydrazine derivative meSCSnmenH
2
and variants
were found to produce both Tcn and Ren cores directly from the tetroxometallates in high yields [130, 131]. Although no
intermediates could be isolated with Tc, [Reo(nHnmeCSSme)
2
]
+
can be isolated from the reaction of [Reo
4
]
−
with the
n-methylated hydrazine in the presence of HCl and can be subsequently be converted to [RenCl
2
(PPh
3
)
2
] by addition of
PPh
3
[132] or [Ren(dtc)
2
] (dtc = dithiocarbamate) by adding dtc [133]. If PPh
3
is added to the starting [Reo
4
]
−
/hydrazine
combination, no intermediates are accessible en route to the nitride, but removal of the hydrazinic methyl group permits
the isolation of the chelated hydrazide
38,
which can subsequently be degraded to the nitride [134]. The meS group is not
a prerequisite for nitride formation and thiosemicarbazide also gives [RenCl
2
(PPh
3
)
2
], but here two consecutive intermedi-
ates are isolable: a chelated hydrazide analogous to
38
and [Re(nH)(nHnH
2
)Cl
2
(PPh
3
)
2
] [135]. This chemistry rather well
illustrates both the subtleties of the product dependence on hydrazine substituent and also the greater kinetic stability of
rhenium permitting the isolation of intermediates (Figure 6.13).
2-
N
N
N
N
PhMe
2
P
Tc
Re
S
N
S
Re
S
NC
CN
S
S
PhMe
2
P
PPh
2
N
Ph
2
P
S
S
Cl
S
N
S
CN
NC
Ph
2
PPh
2
N
34
35
36
fIgure 6.11
Technetium and rhenium(V) nitrido complexes with sulphur-containg ligands.
N
P
Ph
3
Tc
S
S
N
N
Cl
S
Re
S
S
N
N
Cl
S
PPh
3
O
O
TcNOET
38
37
fIgure 6.12
TcnoET and a nitride complex precursor.