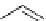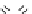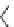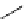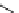Chemistry Reference
In-Depth Information
-
Cl
Cl
Ph
O
O
N
N
S
Na
+
N
O
O
Cl
N
N
Ph
Cl
Cl
Iodogen
®
Chloramine-T
Cl
O
N
OH
O
O
O
N-chlorosuccinimide
Peracetic acid
fIgure 5.17
Common oxidation reagents used in radioiodination chemistry, typically producing I
+
Cl
-
.
5.8.3
124
Iodine radioiodination protocol
unlike radiometals such as
86
Y/
68
Ga/
64
Cu/
89
Zr, radioiodine is not utilised through chelation, instead requiring covalent
attachment to a molecule. Common radioiodination techniques include electrophilic substitution of various leaving groups
via reactions such as iododeprotonation (R-h), iododediazonisation (R-n
2
), iododeboronation (R-B(Oh)
2
), iododestannyl-
ation (R-sn(Me)
3
), iododesilation (R-si(Me)
3
), and iododethallation (R-Tl(OCOCF
3
)
2
) [178]. Iodogen
®
[194], chloramine-T
[195], n-chlorosuccinimide, iodine monochloride (ICI) [196], peracetic acid, and various metal ions (Tl
3+
, Ce
4+
) are all
common iodination reagents used in radioiodination reactions (Figure 5.17) [27, 178].
Many of these reagents generate hOCl, which is an oxidising agent. They generate I
+
cations
in situ
by oxidation of
radioiodide anions, which can then directly radioiodinate activated aromatic rings through electrophilic substitution, such as
demetallation of a good leaving group (i.e., organoboranes, si, Ge, sn, hg, Tl). Generation of I
+
(usually forming I
+
Cl
-
when
generated by
N
-Cl containing oxidising agents) allows for possible quantitative radioiodination, where I
2
labelling would
only offer a theoretical maximum yield of 50% [33]. Of the organometallic precursors, organostannanes such as tributyltin
are most common. nucleophilic exchange reactions do not utilise these oxidising reagents because they require the native
I
-
obtained from [
124
I]-naI.
Figure 5.18 illustrates several examples of
124
I-radioiodination protocols, showing examples from currently used radio-
pharmaceuticals. nucleophilic exchange reactions are shown as halogen exchange reactions that utilise various metal cata-
lysts, with activated aromatic rings providing the best reaction kinetics (level of ring activation also influences
in vivo
stability). The most typical catalysts used for nucleophilic exchange reactions are ammonium sulphate or copper(II) salts
[197]. Electrophilic substitutions are the most common radioiodination reactions and are shown using oxidising agents such
as Iodogen
®
or chloramine T (Figure 5.18). The
124
I is oxidised
in situ
to I
+
, followed by direct (radioiododeprotonation) and
organometallic (radioiododemetallation) electrophilic substitution. Prosthetic groups are also used (similar to
18
F chemistry)
that are first radioiodinated (electrophilic substitutions shown in Figure 5.18) followed by conjugation to a biovector through
standard coupling methods (e.g., amide couplings).
Examples given for
124
I nucleophilic substitution are
124
I-IAZGP (
124
I-IAZAG) [186, 198],
124
I-dRFIB [199], and
124
I-MIBG
[180]. The radiotracer 1-(2-deoxy-β-d-ribofuranosyl)-2,4-difluoro-5-methylbenzene (
124
I-dRFIB) is a non-hydrogen
bonding thymidine analogue used to study the importance of nucleoside base hydrogen bonding in dnA replication and cell
proliferation [199]. Radiosynthesis of the compound
124
I-FIAu is shown as an example of an iododeprotonation electrophilic
substitution [182, 183], and
m
-[
124
I]-iodophenylpyrrolomorphinan (
m
-[
124
I]-IPPM) as an iododestannylation reaction utilising
a trimethyltin 'cold' precursor [200].
m
-[
124
I]-IPPM is a delta opioid (dOP) receptor agonist used for studying pain treatment
[200]. Both of the examples shown in Figure 5.18 for
124
I-labelled prosthetic groups utilise electrophilic radioiodination
reactions. [
124
I]-I-hPP-VG76e is an anti-VEGF diabody conjugated to the Bolton-hunter reagent
124
I-shPP for imaging
tumour angiogenesis [201]. The
124
I-shPP prosthetic group has also been conjugated with an anti-hER2 diabody, commonly
used for imaging breast cancer [202]. The
124
I-sIB prosthetic group conjugate m-[
124
I]-IBA-Annexin-V utilises as a biovector
the 36 kda protein Annexin-V, which is used for studying cell apoptosis in tumours [203-205]. Annexin-V provides targeting
access to the early stages of apoptosis, as Annexin-V has a strong affinity for phosphatidylserine (Ps), which is translocated
from the inner to the outer membrane at the onset of apoptosis [203-205]. An elegant class of reactions directly label
tyrosine residues on proteins and antibodies (i.e., anti-CEA minibodies and diabodies, Annexin-V, A33 monoclonal anti-
body) without the need for prosthetic group conjugations [202]. It must be considered that the activated aromatic rings used
for direct protein labelling, such as the phenol group found in tyrosine, can have stability issues and are more susceptible to
in vivo
deiodination [33].