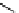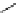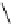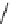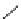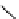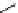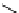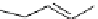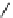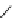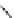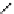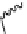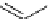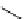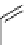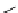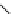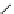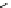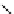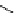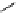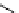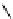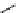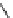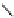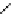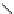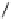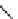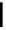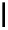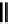Chemistry Reference
In-Depth Information
CO
2
H
HO
2
C
CO
2
H
HO
2
C
NCS
N
N
N
N
NCS
N
N
N
N
CO
2
H
HO
2
C
HO
2
C
CO
2
H
p-NCS-Bn-DOTA
p
-NCS-Bn-DOTA
HO
2
C
CO
2
H
HO
2
C
HO
2
C
CO
2
H
N
N
O
N
N
N
R
H
N
N
N
N
N
N
N
HO
2
C
HO
2
C
HO
2
C
CO
2
H
CO
2
H
CO
2
H
HO
2
C
HO
2
C
DOTAGA
DOTASA
BCNOT-monoamide
fIgure 5.7
Bifunctional dOTA derivatives that retain full octadentate coordination, and the novel nOTA-BFC BCnOT-monoamide
(nETA) that expands its coordination sphere from 6 to 7 coordinate (8 including amide oxygen,
r
= biovector) [45, 63-65].
Every metal ion will coordinate with a given ligand differently, and therefore can form different isomers in solution.
These isomers often have a temperature-dependent fluxional behaviour, which can be studied by variable temperature (VT)
nMR [63, 65]. VT nMR experiments have been performed with In(III) and Y(III) complexes of dOTA [63, 65], which dem-
onstrate this fluxional behaviour in solution, as well as the difference in coordination environment between the two metal
complexes. Chelators that exhibit the lowest degree of isomerism with a given metal ion are generally preferred, because
they tend to be more inert [45, 62]. The modified nOTA chelator BCnOTA (nETA, BCnOT-monoamide as the BFC
derivative, Figure 5.7) is promising for use with Y(III), because it has been reported to have labelling kinetics as fast as
acyclic chelators, as well as a high degree of stability and rigidity imparted by the macrocyclic framework [66].
Because
90
Y in practice is strictly a β
-
emitter, it must be used in conjunction with a sPECT or PET isotope such as
111
In,
86
Y, or
89
Zr in order to perform imaging studies and collect quantitative location data (typically combined with CT/MRI
data). This provides crucial information on organ uptake kinetics and radiochemical complex stability, identifies dose-limit-
ing organs (often kidneys, bone, or liver depending on mode of excretion and metabolism), and allows for dose estimates to
various organs to be calculated (dosimetry) [67]. This allows clinicians to calculate the amount of
90
Y to administer to a
patient while minimising toxicity to the bone marrow and kidney, often the most problematic and radiosensitive organs to
90
Y [67]. The combined use of two isotopes for imaging and therapy is referred to as a matched isotope pair, or a theranostic
agent [20]. The goal of internal radiation therapy is to maximise the amount of radioactive dose delivered to a biological
target (i.e., tumours), minimise localisation in non-target tissue, spare organs from damage, and have fast blood clearance
and excretion of any non-target-bound radiopharmaceutical. This highlights the importance of accurate dosimetry, which
relies on a close match in chemical and biological properties in a BFC-based radiopharmaceutical when coordinated with
two different isotopes [1-6]. Problems arise here because, as previously discussed, different metal ions have different pref-
erences for coordination number, geometry, and donor types, and often exhibit different behaviour
in vivo
[1-6]
.
These dif-
ferences can result in a lack of bioequivalence, which causes different degrees of organ uptake and decreases the accuracy
and predictive power of dosimetry techniques [1-6]. For these reasons, the matched isotope pair of
86
Y and
90
Y is ideal,
because they form chemically identical chelate complexes [1-6].
When chelate complexes are coupled to peptides, they can significantly affect biodistribution and receptor binding, but
when looking at large biovectors such as antibodies, the change is relatively small, because the antibody is massive (~150 kda
for an intact antibody) relative to the chelate complex [68]. Work performed with a 1B4M-dTPA antiTac monoclonal anti-
body conjugate demonstrated that the differences in biodistribution between
111
In and
90
Y complexes was between 10-15%,
with bone marrow uptake being underestimated by
111
In biodistribution data [68]. The story changes when working with
smaller peptide biovectors (bombesin, RGd, octreotide, etc.); in these cases, different metal ions and coordination modes
can impart significantly different properties to the complex and result in larger differences in tissue distributions and receptor
binding affinities [67, 69].
using a matched isotope pair such as
111
In (γ) and
90
Y (β
-
) is not ideal if the two complexes are not sufficiently similar in
their coordination spheres and properties. The availability of
86
Y as a positron emitter allows for high-resolution PET imaging
and dosimetry, while retaining identical chemistry to
90
Y and providing a true matched isotope pair [1-6]. PET imaging and
dosimetry performed with
86
Y-ChX-A″-dTPA-trastuzumab as a surrogate for
90
Y therapy has demonstrated its superior