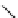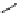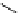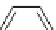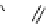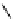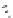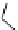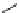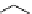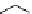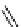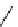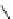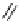Chemistry Reference
In-Depth Information
R
R
S
S
HN
HN
HN
HN
HO
2
C
NNN
CO
2
H
CO
2
H
HO
2
C
NNN
HO
2
C
CO
2
H
HO
2
C
CO
2
H
CO
2
H
CO
2
H
CHX-A″-DTPA
CHX-B″-DTPA
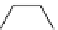
fIgure 5.6
Isomers of the modified dTPA chelator ChX-dTPA, with ChX-A″-dTPA being the most stable isomer and having
improved kinetic inertness
in vivo
over dTPA (
r
= biovector) [42].
faster reaction kinetics that can label some radiometals quantitatively at room temperature over a short period of time; how-
ever, it is not nearly as stable
in vivo
as its macrocyclic counterparts [42]. This shortcoming could be improved through
design of novel acyclic chelators or by modification of well-known acyclic chelators such as the dTPA derivative ChX-A″-
dTPA (Figure 5.6) [42].
Although dTPA and dOTA are the most commonly investigated chelate systems for Y(III), the chelator ChX-A″-dTPA
shows significantly improved stability versus dTPA, but it still may not be as stable as dOTA [42]. The cyclohexyl back-
bone of ChX-A″-dTPA makes the chelator more rigid and imposes a degree of preorganisation on the metal ion binding
site [42]. Conjugation through functionalisation in the chelate backbone (Figure 5.6) does not interfere with the metal ion
coordination sphere and has been shown to yield much more stable complexes
in vitro
and
in vivo
than does amide conju-
gation to one of the carboxylate arms [42, 60]. due to the success of antibody biovectors, developing acyclic chelators with
fast metal ion coordination kinetics, as well as high stability and kinetic inertness comparable to that of macrocycles like
dOTA, would be ideal.
An important lesson learned from the example of ChX-A″-dTPA (Figure 5.6) is that the ChX-B″-dTPA isomer is much
less stable
in vivo
with Y(III), although
in vitro
stability assays suggested they were identical [42, 61]. If ChX-dTPA is used
as a racemic mixture it would result in significant decomposition and radio-demetallation
in vivo
[42, 61]
.
The metal-chelate
portion of a radiopharmaceutical ideally does not interact with the target receptor, and often there is a spacer group placed
between the biovector and the metal-chelate complex to minimise any interference. Considering this, one might assume that
having different isomers of the metal-chelate complex should not affect receptor binding or biodistribution, because it is not
the radiometal complex that interacts with the target receptor. This proposition assumes that there is sufficient space between
the radiometal complex and biovector and that the chemical properties such as coordination number, geometry, and polarity
are the same between isomers. This is an oversimplified view and although it appears to be true according to many
in vitro
assays, it turns out to be inaccurate
in vivo
, as demonstrated by the different
in vivo
stabilities of ChX-A″-dTPA versus
ChX-B″-dTPA [42, 61]. Multiple isomers of a BFC-metal complex can exhibit different pharmacokinetic properties, poten-
tially leading to differential rates of decomplexation depending on organ distribution (Figure 5.2) [45, 62]. These examples
demonstrate why the stereochemistry of chelators and their metal ion complexes should be fully investigated. It should be
noted that the studies performed above, comparing the different isomers of ChX-dTPA, were performed with isotopes of
Y(III) [42, 61], thus the results may vary with different radiometals, although the same principles apply.
The site of bioconjugation on the BFC must be chosen carefully because it can compromise the metal ion coordination
sphere. For example, the carboxylate arm functionalised dOTA derivatives interfere with metal ion coordination by blocking
one of the coordinating carboxylate arms (although the amide carbonyl groups can still weakly coordinate, Figure 5.5) [45].
several dOTA derivatives have been synthesised that alleviate this problem through carbon backbone and side-arm function-
alisation, for example, dOTAGA, dOTAsA, and various isothiocyanate derivatives (Figure 5.7) [45, 63, 64].
These dOTA-BFC derivatives retain full denticity (octadentate), as well as the same thermodynamic stability and kinetic
inertness as dOTA [45, 63, 64]. side-arm and backbone functionalisation provide sites for biovector conjugation that do not
disturb radiometal coordination; however, as with dOTA, radiometallation conditions still require heating and therefore are
not ideal for antibody conjugations [58, 59]. The rigid pre-organised metal ion binding sites in macrocycles provide very
favourable bonding interactions (macrocycle effect) [44], and often result in fewer isomeric species being present in solution
[63]. The chelator must be carefully matched with the specific radiometal; as an example of chelate-radiometal specificity,
dOTA is the most kinetically inert chelator for Y(III); [42] however, the trend is reversed for the similarly sized metal ion
lu(III), where dOTA is actually found to be less inert to acid decomplexation than is the acyclic chelator ChX-A″-dTPA
[40]. This example stresses the importance of carefully matching the chelator with the metal ion and that stability trends
evaluated with a specific metal ion and a set of chelators are not universal and often do not translate to other metal ions.