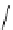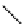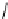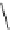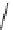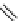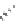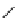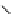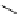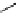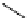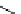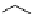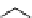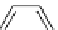Chemistry Reference
In-Depth Information
NH
2
NCS
Antibody (i.e: trastuzumab)
NH
2
HN
Or
NH
N
N
N
H
2
N
HO
2
C
CO
2
H
64
Cu /
68
Ga /
86/90
Y /
89
Zr
HO
2
C
CO
2
H
CO
2
H
O
O
H
CHX-A"-DTPA
NH
HN
Bifunctional Chelator
O
β
+
(PET)
NH
HN
HO
O
NCS
O
CO
2
H
β
-
(Therapy)
Peptide [i.e:
cyclo
(Arg-Gly-Asp)]
γ
(SPECT)
Biovector
H
2
N
fIgure 5.1
Illustration of a BFC-based radiopharmaceutical agent conjugated to a biological targeting group (biovector).
exhibit different biodistribution profiles and binding activity. Effort should be channelled into designing a chelate system
that is modular and easily modified for different linker types so that different bioconjugation techniques can be used. The
biodistribution profile of a compound and its interaction with a conjugated biovector can be tuned by changing the polarity
and charge of the chelate (the degree of polarity can be assessed by octanol-water partition coefficients (log P)), thus a
modular design containing easily modified synthons is important for optimising these properties. It is most common to
conjugate a BFC to a biovector prior to radiolabelling with a radiometal, which is an ideal method for clinic-ready kit for-
mulations. There are many excellent and extensive review articles available that elaborate on different aspects of these inor-
ganic PET isotopes and their incorporation into radiopharmaceuticals, as well as many of the other concepts discussed in
this chapter [12, 18, 19, 21-33].
5.3
radIopharmaceutIcal staBIlIty
The purpose of a bifunctional chelating agent is to sequester a radiometal ion with such high thermodynamic stability and
kinetic inertness that it is not released through any mechanism under physiological conditions (decomplexation, acid-
catalysed dissociation, trans-chelation by serum proteins, protonation, trans-chelation by bone, complex adsorption to
bone, hydrolysis, etc.). The result of radiometal loss from a radiopharmaceutical
in vivo
is non-targeted distribution of the
radiometal to various compartments in the body, depending on the biological interactions of the specific radiometal. Each
metal ion has its own unique properties to contend with when attempting to incorporate it into an imaging/therapeutic
agent, such as its aqueous chemistry, redox chemistry, and affinity for native biological chelators.
When evaluating chelators for use in radiopharmaceuticals, kinetic inertness
in vivo
is a much more important factor than
absolute thermodynamic stability of the metal-chelate complex (Figure 5.2). Thermodynamic stability constants (
K
Ml
= [Ml]
/ [M][l]) can be calculated from experiments such as potentiometric and/or spectrophotometric titrations. These thermody-
namic stability values can be useful as preliminary comparisons of the efficiencies of various chelators for a particular metal
ion [17, 34]. stability constants give a number for the direction and magnitude of the equilibrium in a metal-chelate
coordination reaction; however, they give no rate information, thus the kinetics of dissociation must be probed through other
methods (Figure 5.2) [35, 36].