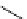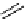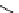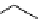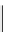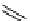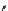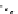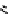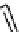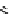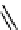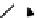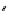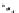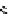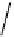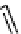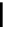Chemistry Reference
In-Depth Information
BH
3
.
11
CO
or
[CuTp*(
11
CO)]
O
I
H
2
N
*
H
+
or
xenon transfer
11
CO
Pd Cat.
scheme 4.20
Low pressure
11
CO reactions. synthesis of the model compound [
carbonyl
-
11
C]
N
-benzylbenzamide via palladium-
mediated carbonylation using the BH
3
.
11
CO adduct, [Cu(Tp*)
11
CO] complex or
11
CO xenon transfer method.
H
H
K
+
-
B
B
11
CO, He
N
N
N
N
N
N
N
N
N
N
CuCl
N
N
Cu
11
C
O
scheme 4.21
Formation of [Cu(Tp*)
11
CO] via the complexation reaction of potassium tris(3,5-dimethylpyrazolyl)-borate (Tp*),
CuCl and
11
CO.
analogue. Excellent rCy of model amides were achieved (scheme 4.20); however, the yields were found to be highly
dependent on the Pd catalyst system [116]. A third low pressure
11
C carbonylation method has now been recently developed
by Eriksson et al. [117] that exploits the high solubility of xenon gas in organic solvents to efficiently transfer
11
CO to a small
volume reaction vial. Because xenon is highly soluble in organic solvents such as THF and toluene typically used for metal-
mediated coupling reactions, it can be used to transfer
11
CO into small volumes of reagent solution without a build-up of gas
pressures. The method has been demonstrated to quantitatively transfer
11
CO from a silica trap into solvent volumes of less
than 1 ml without significant pressure build-up and has been used to perform several model Pd- and rh-coupling reactions
for the formation of labelled amides, ureas, and esters. This method is particularly interesting because it avoids the use of
additional trapping reagents, thus making it a chemically much simpler protocol for
11
CO labelling (scheme 4.20).
Microfluidic systems have been used for performing low pressure palladium mediated C-11 carbonylation reactions of
aryl halides. One system exploits a silica-supported palladium catalyst that was packed into PTFE tubing, effectively acting
as a mini-packed bed reactor [105] (Figure 4.2). A high surface area-to-volume ratio is generated within this device owing
to the large surface area of the silica supported catalyst material. This improves the interfacial gas-liquid contact between the
11
CO/He gas stream and the aryl halide/amine coupling reagents in solution. A series of different C-11 amide systems could
be effectively labelled in good to excellent rCy. Advantageously, the method is technically straightforward but requires a
low temperature
11
CO trapping step prior to reaction in the microfluidic device. The micro-tube reactor loops could be reused
for successive labelling reactions for the synthesis of the same labelled compound, changing to a different substrate using
the same reactor resulted in mixtures of labelled products. More recently, a glass fabricated device has been used to perform
11
CO carbonylation labelling reactions. The device contains two inlet ports for gas and liquid reagents, a 5 m long reaction
channel and an outlet port. The microchannels were chemically etched, have a semicircular cross-sectional profile, and are
220 μm wide and 100 μm deep. The main reaction channel, which is 5 m long, occupies most of the 90 x 15 mm footprint
area. The long residence channel was necessary to give sufficient residence times of the gas and liquid reagents on the device
under gas-liquid annular type flows. A typical
11
CO labelling process, involving a
11
CO pre-trapping stage, reaction on the
device, and a flushing step is complete in less than 15 min. A range of simple amide and ester molecules were labelled with
11
CO in good rCys using [PdCl
2
(xantphos)] as catalyst.
Besides palladium-mediated reactions, rhodium-mediated carbonylation reactions have also been used for the synthesis
of a range of C-11 carbonyl containing compounds, including malonates [118], hydroxyureas [119], carbamates [120], and
diphenyl ureas [120]. in the presence of an azide, the rhodium catalyst enables the insertion of
11
CO forming rh-complexed