Environmental Engineering Reference
In-Depth Information
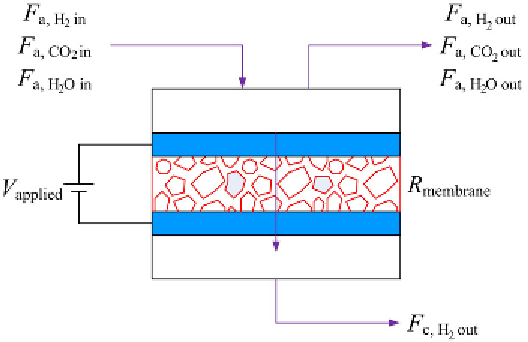
FIGURE 5.5
An example of the polymer electrolyte hydrogen pump (PEHP).
Source
: Reproduced with
permission from Abdulla et al. [9]. (See color insert.)
membrane to the cathode, where they are reduced back to H
2
. The pumping
is done at low potentials, and only hydrogen is pumped across the electrolyte
and membrane. The PEHP acts both as a separator unit and as a pump.
5.3 LIQUID HYDROGEN
Liquid hydrogen has a much higher volumetric capacity compared with gas
hydrogen as shown in Section 5.1. It is expected that if hydrogen is stored
in liquid phase, it will maintain high energy content, which could meet the
DOE target. In fact, liquid hydrogen has been used in some special applica-
tions such as the Space Shuttle. However, liquid hydrogen only exists in a
very narrow temperature and pressure range as shown in the phase diagram
in Figure 5.6: between the triple point 13.8 K (at this temperature, gas, liquid,
and solid phases coexist) and critical point 32.97 K (the heat of vaporization
is zero at and beyond this temperature). Hydrogen has the second lowest
boiling point (transition temperature from liquid to gas), 20.28 K, and melting
point (transition temperature from solid to liquid), 14.01 K, of all substances,
second only to helium. The boiling point is a critical parameter since it
defines the temperature to be cooled in order to store and use the fuel as a
liquid. Thus, liquid hydrogen requires cryogenic storage and boils around
20.28 K (−252.87°C or −423.17°F). Cooling to such low temperature
requires a fairly amount of energy, and storing the liquid hydrogen needs
specific containers and management.
Search WWH ::

Custom Search