Environmental Engineering Reference
In-Depth Information
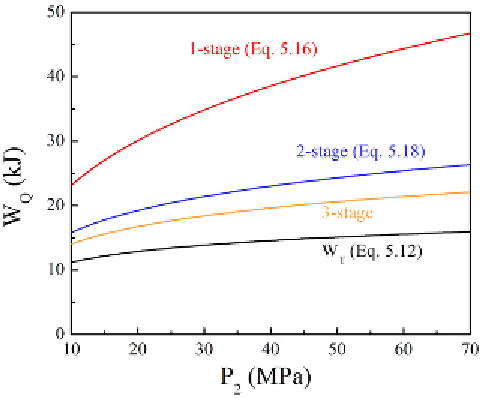
FIGURE 5.4
Energy required to compress 1 mole hydrogen from 1 atm at 20°C for a one-stage, a
two-stage, and a three-stage compression process. The lowest curve show
W
T
calculated using Equation
(5.12) for the ideal gas case. (See color insert.)
approximated by a polytropic process. The index
γ
in Equation. (5.16) and
Equation (5.17) can be replaced by the polytropic index
β
(≥
γ
) with
ηγ
ηγ
β
=
,
(5.19)
1
+ −
γ
while keeping the similar formula for Equation (5.14), Equation (5.15),
Equation (15.16), Equation (5.17), and Equation (5.18). Here,
η
is the poly-
tropic efficiency.
There are other issues associated with the compressed hydrogen storage,
in particular, cost and safety. The cost includes that for the lightweight tank
and for the compressor as well as the energy required for compression. Novel
compression technique with low cost and low energy requirement is also a
challenge issue and active topic for high pressure hydrogen storage. For
example, recently, the electrochemical hydrogen pumps based on the devel-
opment of polymer electrolyte membrane fuel cell has been proposed and
studied extensively [8]. An example of polymer electrolyte hydrogen pump
(PEHP) is shown in Figure 5.5 [9]. It consists of a proton-conducting polymer
electrolyte sandwiched between two porous electrodes. Gas containing H
2
is fed to the anode. Under an applied voltage, H
2
is oxidized into protons
and electrons. The protons can transport across the polymer electrolyte
Search WWH ::

Custom Search