Biomedical Engineering Reference
In-Depth Information
improved spatial distribution. Thus, fl uid
fl ow-induced shear forces clearly provide
important biological stimulation of osteoblas-
tic cells residing in three-dimensional metal
scaffolds.
A level of shear stress in the range of
10
dynes/cm
2
appears suffi cient to stimulate
osteoblasts [
2
to
], i.e. by increased secretion
of nitric oxide and prostaglandin E
2
after only
a short period of exposure to fl uid shear stress.
It should be noted that exposure of cells to high
levels of shear stresses may cause cell detach-
ment or damage.
1
,
17
,
19
5.4.3 ECM Proteins
Cellular interactions with the ECM are thought
to orchestrate tissue organization by regulating
cell differentiation and function. The ECM pro-
duced by osteoblasts is complex and consists of
several different classes of molecules that regu-
late the modeling and remodeling of bone. The
ECM contains structural components, includ-
ing type I collagen and fi bronectin, as well as
proteases that degrade the matrix. The ECM
also serves as a reservoir for growth factors,
including members of the transforming growth
factor
) superfamily. These compo-
nents of the ECM, produced by the osteoblasts,
act alone or in synergy with other factors to
affect cell differentiation and survival by means
of autocrine feedback mechanisms that regu-
late the rate of bone formation.
Surface chemistry and precoating of implant
materials are key components necessary to
establish a proper biomaterial-bone interface.
However, information concerning the behavior
of cells on implants precoated with ECM pro-
teins remains scarce. Several investigations
using type I collagen-coated implants found
that type I collagen enhances proliferation and
accelerates differentiation and mineralization
of osteoblastic cells [
β
(TGF-
β
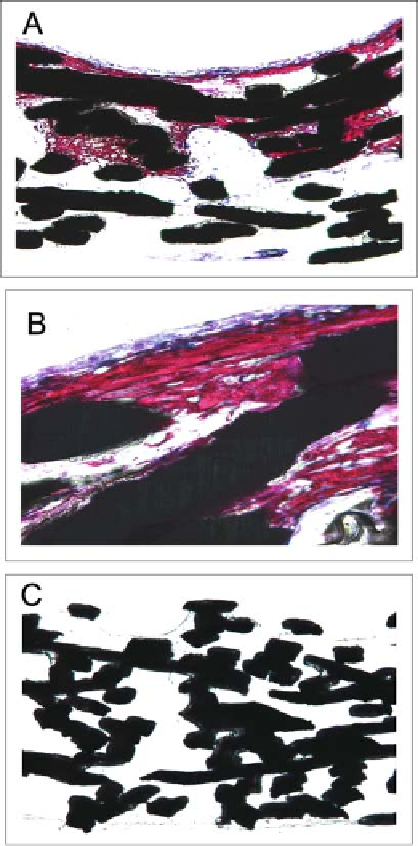
Figure 5.2.
Light micrographs of seeded titanium fiber
mesh after 16 days in culture. The cells were cultured in the
flow-perfusion system (A and B) or under static culture condi-
tions (C). The sections from the flow-perfusion system showed
mineralized matrix throughout the whole meshes covered with
layers of osteoblast-like cells. The sections of the static culture
specimens showed only a thin layer of cells covering the mesh,
and no matrix mineralization was observed.
].
Another ECM protein that may provide
information to osteoblasts during their differ-
entiation is fi bronectin. Fibronectin expression
is highly localized to bone surfaces in vivo and
occurs at the periphery of nodules in vitro [
5
,
20
cells to increasing levels of mechanical stimu-
lation, in the form of fl uid shear stress, whereas
chemotransport conditions for nutrient deliv-
ery and waste removal remained constant.
Increased shear forces produced an enhance-
ment of mineralized matrix deposition and
].
Acting in this way, fi bronectin can support the
recruitment or migration of preosteoblasts.
Furthermore, fi bronectin also may promote
the synthesis and organization of the ECM
produced by osteoblasts that respond to
24