Biomedical Engineering Reference
In-Depth Information
showed that using high cell densities during
inoculation of the scaffolds produced more
uniform results among experimental runs.
Scanning electron microscopy (SEM) exam-
ination showed that for both droplet and cell-
suspension samples, cells were present only at
one side of the mesh. When rotation was used,
no cell sheet was formed, and cells invaded the
meshes and grew to surround the titanium
fi bers.
a titanium fi ber mesh. After seeding, the con-
structs were cultured under static conditions or
in a fl ow-perfusion system for several days (Fig.
5
). Cell proliferation and matrix mineraliza-
tion increased in the fl ow-perfusion system.
Examination by SEM revealed that the samples
subjected to fl ow-perfusion culture were com-
pletely covered with layers of cells and mineral-
ized matrix. In addition, this matrix extended
deep into the scaffolds. In contrast, meshes
cultured under static conditions had only a
thin sheet of matrix present on the upper
surface of the meshes. Evaluation of the light
microscopy sections confi rmed the SEM obser-
vations (Fig.
.
1
5.4.2 Nutritional Conditions
Another variable affecting implant outcome is
the optimization of the nutrient conditions
and oxygen supply so that the osteogenic capac-
ity of the cultured cells is enhanced. Cell cul-
ture in three-dimensional scaffolds occurs
under completely different conditions from
those present in conventional planar two-
dimensional conditions, in which all cells are
continuously exposed to the culture medium.
An inverse relationship between proliferation
and differentiation in bone cell cultures result-
ing from a decline in the nutritional state
during mineralized matrix deposition has been
observed [
).
Subsequent studies examined the infl uence
of fl uid fl ow and fl uid shear forces on cell-
loaded titanium fi ber meshes in a fl ow-
perfusion system [
5
.
2
],
the authors used different rates of fl ow for an
extended period to permit osteoblast differen-
tiation. Histological analysis showed that an
increased fl ow rate produced a more uniform
distribution of cells and matrix mineralization
throughout the scaffold. Also, an increased
fl ow rate produced an accelerated osteoblastic
differentiation pathway. The osteoblast marker
osteopontin appeared earlier, as did the late
osteoblast
2
,
33
]. In the fi rst study [
2
].
Dynamic culturing of cells (bioreactor, rotat-
ing-wall vessel, and spinner fl ask) after seeding
of the scaffolds has been reported to have a
positive effect on cell proliferation and differ-
entiation. Furthermore, Goldstein et al. [
26
differentiation
event,
calcium
deposition.
In a second study [
], these researchers kept
the fl uid fl ow rate constant, but cultured the
cell-loaded titanium fi ber meshes in the fl ow-
perfusion system using culture media of differ-
ent viscosities. This strategy exposed cultured
33
]
demonstrated that use of fl ow-perfusion tech-
niques enhanced the early differentiation and
three-dimensional distribution of marrow
stromal cells seeded on poly(DL-lactic-co-
glycolic acid) scaffolds in comparison with
scaffolds cultured in a spinner-fl ask bioreactor,
in a rotating-vessel bioreactor, or under con-
ventional static conditions. Static cultured con-
structs exhibited uneven cell distribution and
low cellularity in the center of the constructs,
with most cells growing near the periphery of
the construct. In contrast, constructs cultured
under dynamic conditions showed higher cel-
lularity and a more uniform distribution of
cells throughout the constructs. Interestingly,
the production of extracellular matrix (ECM)
was increased when a dynamic culture method
replaced a static method.
Van den Dolder et al. [
10
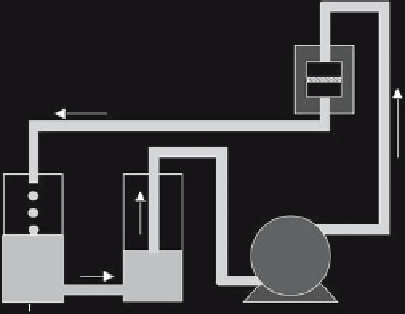
Flow Chamber
with Scaffold
Pump
] investigated the
effect of a dynamic culture method on cell pro-
liferation and differentiation from a seeded cell
suspension of rat bone marrow stromal cells in
36
Media
Reservoirs
Figure 5.1.
Schematic figure of the flow-perfusion system.