Biomedical Engineering Reference
In-Depth Information
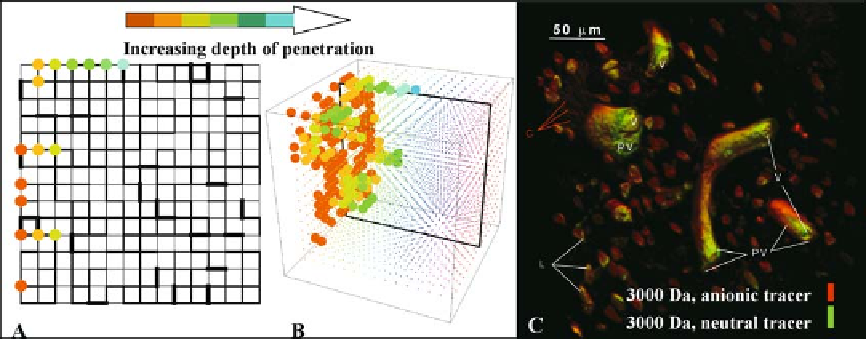
Figure 10.11.
Stochastic network modeling (A, B) as a means to study delivery of drugs and molecular agents in actual micro-
volumes of bone (C). Fig. 10.11C reprinted from
Biorheology
, Volume 40, A.E. Tami, M. B. Schaffler and M. L. Knothe Tate, Probing
the tissue to subcellular level structure underlying bone's molecular sieving function, p. 586, 2003, with permission from IOS
Press.
molecules through the pericellular network
within a defi ned tissue volume (Fig.
included in initial models. The CFD program
was run to calculate the pressure gradient,
fl uid velocity, and maximum shear and radial
stresses imparted to the cell by the fl uid (Fig.
10
). The
model predicts the depth of penetration of
specifi c molecules, and the predictions can be
validated experimentally with the aid of fl uo-
rescently tagged molecules. The predictions
apply to the perivascular space (PV, Fig.
10
.
11
). The model predicted that osteocytes are
subjected primarily to sustained hydrody-
namic pressure and low stresses, whereas cel-
lular processes are subjected primarily to shear
gradients [
.
12
),
the lacunar pore (L), and canaliculi (C) and
can be validated experimentally in scaled-up
models that are produced by stereolithographic
methods.
10
.
11
]. Increasing the number of cana-
liculi in the virtual model had a minimal effect
on the magnitudes of pressure and stress.
Because these effects cannot currently be mea-
sured at the cellular level, a computational
model becomes essential for engineering
design, as in the development of scaffolds,
where cell recruitment, migration, and adhe-
sion are essential.
Obviously it is important to check the valid-
ity of the assumptions that have gone into
model construction. Since the CFD program
uses the Navier-Stokes equations as the govern-
ing equations for fl ow fi eld calculations, the
validity of the
continuum assumption
underly-
ing the Navier-Stokes equations was tested to
ensure that the approach was appropriate at the
length scale of our system. Validation studies
have shown that the simulation is appropriate
to lengths of approximately
1
10.8 Cell to Subcellular Scale
Yet another modeling approach lends itself to
study of the mechanobiological effects of solid
and fl uid interactions in bone. Specifi c compu-
tational fl uid dynamics (CFD) programs
have been developed to study mechanics and
transport in nano- and microelectromechani-
cal systems. We utilized such a program to
develop a computational model of an osteocyte
in situ to understand the mechanical milieu of
the cell and the role of fl uid fl ow in mechano-
transduction from the system as a whole to the
cellular level. Fluid fl ow was explored at the
length scale of the cell by developing a model
of the fl uid space around an osteocyte (Fig.
10
nm, a length that
is just below the minimum postulated dimen-
sion of the annular fl ow channel that surrounds
the osteocytes [
10
.
12
). Flow through the microporosity was not
1
].