Biomedical Engineering Reference
In-Depth Information
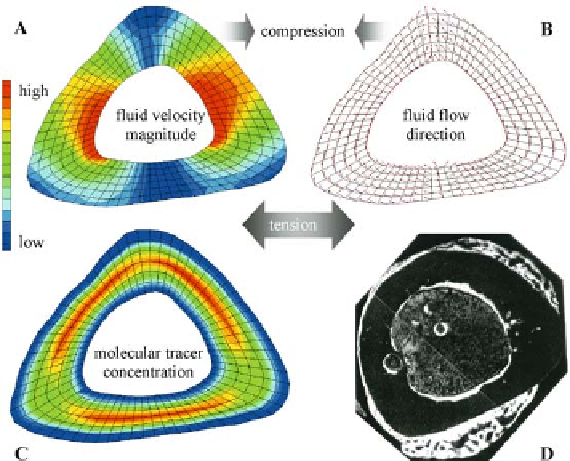
Figure 10.9.
Areas if endosteal bone
apposition in the four-point bending
model of the rat tibia (D) correspond to
areas of increased molecular tracer con-
centration (C) rather than areas of
highest fluid velocity magnitudes (A) or
specific fluid flow directions (B). Fig.
10.9A-C reprinted from
Journal of Theo-
retical Biology
, Volume 2003, R. Steck, P.
Niederer, and M. L. Knothe Tate, A finite
element analysis for the prediction of
load-induced fluid flow and mechano-
chemical transduction, p. 254 (Fig.
10.9B), p. 255 (Fig. 10.9A and C), 2003,
with permission from Elsevier. Fig. 10.9D
reprinted with the permission of Charles
Turner.
areas of endosteal bone apposition colocalized
with areas exposed to higher concentrations of
molecular tracer and not to areas of high fl uid
velocity
tions that depend on site-specifi c material defi -
nitions became apparent. This simulation
guided future studies along two paths, fi rst,
there was a need for better defi nition of material
parameters in our models, i.e., the need to make
them also site-specifi c; and second, the poro-
elastic approach in which the whole cross-
section was treated as a continuum needed to be
readdressed. Our solution was to build a discrete
model of bone at the tissue level to defi ne locally
relevant effective permeabilities that could later
be implemented in the continuum model.
magnitudes
or
specifi c fl uid fl ow
directions.
The power of computational modeling to elu-
cidate biological systems is illustrated by a para-
metric study evaluating how defi nitions of
site-specifi c material properties may infl uence
model predictions (Fig.
). Because a reliable
experimental method has only recently been
reported [
10
.
8
], the value of predictive modeling
becomes compelling: using predictive models,
we can determine which system parameters
infl uence relevant biological effects. This, in
turn, helps set priorities in planning experimen-
tal studies. One parametric model (Fig.
5
10.7 Tissue to Cell to
Molecular Scale
)
accounted for concentric layers of bone that
show differences in porosity, void ratio, and
permeability in three dimensions. Histological
examination provided the rationale for this
model as follows: in cross section, rat cortical
bone exhibits “zones” or concentric layers that
form as the bone grows and that show marked
differences in the number of vascular canals and
cells and in matrix density. We hypothesized
that these differences would infl uence the distri-
bution of pore pressures in the cross section and
thereby infl uence transport through the bone.
W hen pa r a me ter s were v a r ie d by order s of m a g-
nitude and the corresponding pore pressures
across the model sections were calculated,
obvious differences in pore pressure distribu-
10
.
8
Changes in local and tissue-level permeability
infl uence the transport of nutrients and waste
products to and from the osteocytes, as well as
the transport of signaling molecules through-
out the bone cell network. These changes in
permeability are caused in part by changes in
the pericellular transport network that result
from aging and disease. Due to the inherent
limitations of the continuum approach, and
because our goal was to determine site-specifi c
permeabilities as input parameters for an
organ-tissue-level continuum model, we looked
for an alternative approach in building discrete
models that were virtual representations of