Biomedical Engineering Reference
In-Depth Information
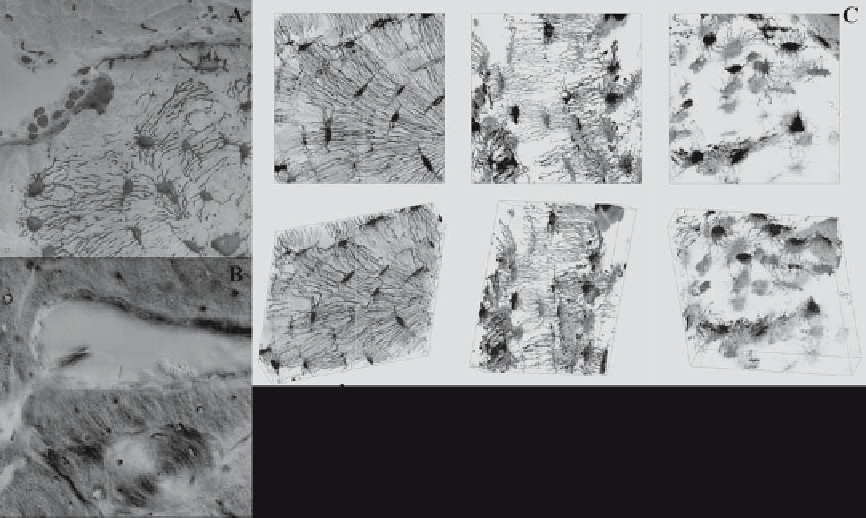
Figure 10.3.
Bone remodeling processes histologically visualized at the tissue and cellular levels. Although histology is helpful for
observing the physiology of the system at one moment in time, remodeling and adaptation are inherently dynamic. Hence, it is impos-
sible to understand the dynamics of remodeling from a static image depicting one point in time. (A) After 20 weeks of immobilization,
osteoclasts resorb the surface of the bone, as evidenced by the crater on the top right edge of the cross section of the ulna. Upon
remobilization, osteoblasts subsequently lay down new osteoid, which fluoresces highly in its unmineralized state (infilled crater:
osteoblasts are visible along the upper edge of the bone). The osteocyte network is observable across the tissue, linking every cell in
the tissue with the blood supply, with bone surfaces to which forces are imparted, and with the marrow cavity. (B) Further into the
cortex, remodeling is observed as a classic cutting cone. Because this image is taken after remobilization, osteoblasts are observable
along the edges of the cutting cone. Red blood cells are visible in the resorption cavity. As the osteoblasts fill in new bone, an osteon
is formed, as is visible in the same micrograph, orthogonal to the cutting cone in the plane of the image (see osteonal cross section
below the cutting cone). (C) As bone is resorbed and as bone degrades because of aging or disease, the cellular network changes, as
observed through changes in network connectivity, cell shape. and cell size. Reprinted with permission from
Advances in Osteoporotic
Fracture Management
, Volume 2, M. L. Knothe Tate, A. E. G. Tami, T. W. Bauer, and U. Knothe, “Micropathoanatomy of osteoporosis:
indications for a cellular basis of bone disease,” pp. 10, 11, 2002. Copyright 2002, Remedica Medical Education and Publishing.
represent the system appropriately. However,
as soon as the equilibrium is disturbed, the
steady-state assumption no longer applies.
Further, depending on the function to be
addressed, the time scale of the system may
vary from fractions of a second, as in cell sig-
naling, to periods as long as
extracellular matrix, and cell senescence),
but such a bone replacement does not exist.
Rather, normal, healthy bone will be con-
sidered the “gold standard” for design specifi -
cations. For the purposes of this chapter,
we will examine bone and its constituents
within the engineering concept of a control
volume as well as within the context of bio-
logical machines and materials. Furthermore,
we will consider how surgeons harness
Nature's endogenous strategies to replace
and promote healing in missing or failed
bones. Then we will follow up the design goal
with development of computer models, fi rst
modeling actual tissue properties and leading
into the rational design and optimization of
tissue-engineered scaffolds. Finally, we will
discuss the experimental validation of in silico
month, the time
it takes osteoclasts to resorb a cavity and osteo-
blasts to fi ll it in with fresh osteoid (Fig
1
10
.
3
,
Fig
), to months and years, the time it may
take a bone to regain its prior mechanical
strength after fracture.
One can conceive of a bone replacement
that is virtually indestructible yet self-
healing, is fully integrated with the biological
tissue, and is immune to the biological con-
sequences of aging (loss of bone mineral
density, cross-linking of proteoglycans in the
10
.
4