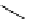Chemistry Reference
In-Depth Information
Table 3.5
Asymmetric allyic alkylation catalysed by the palladium complex of amidine
ligand
OR
CH(CO
2
Me)
2
CH
2
(CO
2
Me)
2
BSA, LiOAc
Ph
Ph
Ph
Ph
3
-C
3
H
5
)Cl], ligand
rt
[Pd(
η
BSA =
N,O
-bis(trimethylsilyl)acetamide
run
R
Pd (mol equiv)
solvent
time (h)
yield (%)
ee (%)
1
COMe
0.05
DCM
48
85
92 (
R
)
2
t
Bu
0.05
DCM
24
99
93 (
R
)
3
t
Bu
0.05
THF
24
91
91 (
R
)
4
t
Bu
0.025
DCM
24
87
94 (
R
)
e
Molar ratio:
Pd/ligand/substrate/CH
2
(CO
2
Me)
2
/BSA/LiOAc
¼
1-5 : 4-20 : 100 : 300 : 300 : 5.
derived from N-substituted diphenylphosphinoacetamides, in which low to moderate
hydrogenating activity and enantioselectivity were obtained [77].
3.3.14.8
Tin
Chiral allylating reagents, readily generated in situ from tin (II) catecholate [Sn(II)
(O
2
C
6
H
4
)], allyl halides, chiral dialkyl tartarates and DBU (1), react smoothly with
R
1
i
Pr
R
2
Me
S
NN
NN
R
3
PPh
3
Me
R
R
1
=
i
Pr, R
2
= R
3
=
Me
R
1
=
t
Bu, R
2
= R
3
=
Me
R
1
=
i
Pr, R
2
+ R
3
=(CH
2
)
n
(n = 4, 5)
R = H, F, OMe
Figure 3.5 Structures of amidine-phosphine and -sulfide hybrid ligand for palladiummediated
couling reaction
Me
Me
N
NMe
2
PPh
3
Fe
Figure 3.6
Structure of ferrocenylphosphine-amidine ligand for palladium coupling reaction