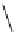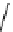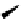Chemistry Reference
In-Depth Information
H
DBU (
1
) (0.8 equiv)
H
Me
O
O
(A) R = Ms
benzene, rt, 1.5 h
O
N
H
90%
RO
O
H
O
E / Z = 87 : 13
H
Me
N
OH
H
O
H
H
CO
2
Bn
Me
O
O
10% Pd-C, H
2
THF
O
N
(B) R = H
H
C
H
O
O
-
NN
Me
Me
HH
Me
Me
NN
Me
+
Me
Me
Me
Me
4
71%
Me
Scheme 3.36
The use of amidines in the
b
-lactam chemistry
crystalline amidinium salts with 4 in the deprotection of the benzyloxycarbonyl function (B
route), respectively [56b] (Scheme 3.36).
3.3.9 Deprotonation
Chiral allyl alcohols are obtained from meso-epoxides by treatment with bases [57-59].
Addition of amidines like DBU (1) alters the reactivity and the enantioselectivity in the
epoxide rearrangements, in which 1 is lithiated and works as a bulk base (a catalyst-
regenerating base) as well as being a strong solvating agent [59] (Figure 3.4). NMR studies
using isotopically labeled chiral lithium amide and lithiated DBU show the formation of a
mixed dimer.
Me
Ph
Me
NN
Li
Li
N
N
Figure 3.4
NMR-supported mixed dimer containing DBU (1)