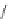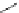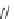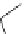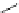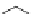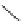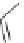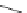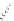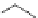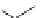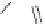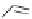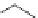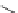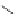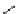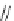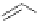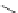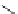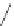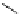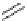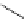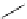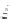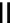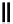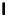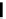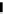Chemistry Reference
In-Depth Information
1. ligand (30 mol%)
Mg(NTf
2
)
2
(30 mol%)
DBU (
1
) (1.5 equiv)
MS 4A, CHCl
3
-78 ˚C , 6 h
ligand
Bn
Me
HO
O
O
NH
Ph
N
+
N
N
O
N
Me
N
N
2. NaBH
4
, THF-H
2
O
Ph
Br
Bn
57% (94% ee)
Scheme 3.33
DBU (1) mediated [3
þ
2] cycloaddition
O
O
O
CO
2
Me
(1 equiv)
N
N
N
N
N
N
-
LiBr (1.5 equiv)
DBU (
1
) (1.5 equiv)
MeO
2
C
N
N
NH
CO
2
t
Bu
CO
2
t
Bu
CO
2
t
Bu
O
O
O
R
R
Li
+
R
MeCN, rt, 3.5 h
R = 2-naphthyl (82%)
R = Ph (86%)
Scheme 3.34
DBU (1) mediated 1,3-dipolar cycloaddition
3.3.7 Dehydrohalogenation
Regioselective introduction of a bromine atom to a double bond in the substituted vinyl
sugar is achieved by bromination with pyridinium tribromide and debromination with
DBU (1) [55] (Scheme 3.35). E-configuration of the product is expected from a specific
anti-addition in the bromination of the E-alkene followed by on E2 (anti-elimination)
process.
3.3.8 Deprotection
In the chemistry of
-lactam antibiotics, isolations of carboxylic acid derivatives are
successfully achieved by formation of amidinium salts [56]. Lewis acid catalysed reaction
of 4-substituted 1-trimethylsilyloxyfurans with 4-acetoxyazetidinone chiron leads to
highly enantioselective construction of tricyclic carbapenam and penems, in which DBU
(1) and Eshenmoser amidine (4) were used for the introduction of the exo double bond on
the
b
b
-lactam skeleton by demesylation (A route) and the isolation of carboxylic acids as
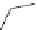
NHBoc
NHBoc
Br
1.pyridinium tribromide
dioxane, rt, 8 h
O
O
NBz
2
NBz
2
EtO
2
C
EtO
2
C
2. DBU (
1
), THF
OO
Me
OO
Me
Me
Me
70%
Scheme 3.35
DBU (1) mediated regioselective introduction of bromine atom to double bond