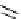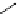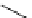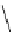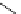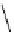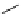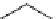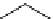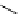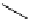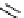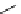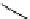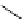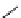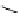Chemistry Reference
In-Depth Information
CN
TsOH
Raney Ni
N
O
N
O
N
N
O
KOH
hydroquinone
NH
3
, MeOH
xylene
N
H
CN
NH
2
88%
84%
DBN (2)
94%
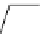
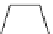
Scheme 3.4
Preparation of DBN (2)
These amidines have been extensively applied to dehydrohalogenation in organic
synthesis and in some cases DBU (1) is more effective than DBN (2) [5]. A double bond
can be also introduced into organic molecules by elimination of sulfonate ester instead of
the halogen atom (i.e. dehydrosulfonation in addition to dehydrohalogenation). Further-
more, these amidines can be applied to the Wittig reaction [6], aldol condensation [6], 1,3-
allyl rearrangement [7] and epimerization of the
b
-lactam skeleton (at C
6
of the penicillic
acid derivatives). Sterically hindered phenols (e.g. 2,6-di(tert-butyl)-4-fluorophenol) are
O-acetylated with DBU (1), which is superior to sodium hydroxide in the synthesis [8].
The related 6-6 bicyclic system, 2,10-diazabicyclo[4.4.0]dec-1-ene (3), was prepared
fromtrans-decahydro-1,8-naphthyridine [9] andanalternativemethodvia direct cyclization
of bis(3-aminopropyl)malonic acid was developed for large scale operation [10]. Heinzer et
al. [11] reported the preparation of a sterically strongly hindered bicyclic amidine, 3,3,6,9,9-
pentamethyl-2,10-diazabicyclo[4.4.0]dec-1-ene (Eschenmoser amidine) (4) (Figure 3.2)
and its N-alkylated analogues and their potential uses in the formations of salts of carboxylic
acids and related proton complexes of bidentate ligands (Section 3.3.8) [11b].
The efficiencies of DBU (1) and DBN (2) as sterically hindered (non-nucleophilic) and
strong organobase catalysts have been widely demonstrated [5]. However, Reed et al. [12]
claimed that they could behave as strong nucleophiles in the reaction of chlorobis
(diisopropylamino)phosphane and DBU (1) or DBN (2).
Modification of the amidine function to chiral versions has been examined. For example,
C
2
-symmetrical chiral bicylic amidine 5 was prepared for studies on molecular recognition
and were proven to differentiate analytically between the enantiomers of chiral carboxylic
acids [13]. Near the same time, a mannose-based amidine 6 was synthesized as a potential
mannosidase inhibitor, but not a chiral auxiliary [14]. Three enantiopure hydroxyl
substituted amidines 7 of the DBN-type were synthesized from 5-(phenylsulfonyl)pyrror-
idine-2-one by an oxazaborolidine-catalysed reductive desymmetrization of meso-imide
followed by functionalization through N-acyliminium ion [15] (Figure 3.3).
Me
Me
Me
H
N
H
N
Me
Me
3
4
Figure 3.2
A 6-6 bicyclic amidine system (3) and the Eschenmoser amidine (4)