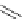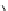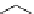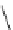Chemistry Reference
In-Depth Information
R
3
R
3
R
3
N
-
N
-
N
R
4
C
R
4
R
4
C
+
C
N
+
R
1
R
1
R
1
N
N
R
2
R
2
R
2
Figure 3.1
Possible conjugation forms of amidine
NO
2
H
H
NO
2
NO
2
N
O
O
+
NH
2
N
O
HO
Me
O
Me
NH
H
Scheme 3.1
Benzamidine-catalysed hydrolysis of p-nitrophenyl acetate
of halotetraene with DBN (2) in benzene gave a conjugated pentaene, in which a newly
introduced double bond has Z-configuration [4] (Scheme 3.3). DBN (2) was found to be the
most suitable dehydrohalogenation reagent among organobases examined.
DBU (1) and DBN (2) are originally synthesized from cyclic lactams by three steps of
Michael addition of acrylonitrile, reduction with Raney nickel, and treatment with p-
toluenesulfonic acid (TsOH). Thus, DBN (2) was prepared in 69% overall yield [4]
(Scheme 3.4).
R
1
R
R
N
H
(a)
R
3
R
N
H
X
R
1
R
1
R
R
2
R
R
N
H
H
N
R
R
3
+
R
3
+
N
X
R
N
H
X
R
R
1
R
2
R
2
N
H
R
R
R
3
(b)
N
H
R
R
2
X
Scheme 3.2
Possible mechanisms for amidine-catalysed allylic rearrangement
Me
Me
Me
Me
Me
Me
Me
n
DBN (
2
)
N
N
OAc
benzene
X
Me
Me
63%
n = 3: DBU (
1
)
n = 1: DBN (
2
)
Me
OAc
Scheme 3.3
Dehydrohalogenation wrih DBN (2) in the synthesis of vitamin A