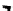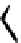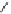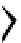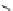Chemistry Reference
In-Depth Information
Table 2.13
Basicity values for proazaphosphatranes
H
H
R
1
H
N
R
3
N
P
H
P
N
R
2
N
N
N
N
N
138H
+
R
1
R
2
R
3
p
K
a
(MeCN)
p
K
a
(DMSO)
p
K
a
(THF)
GB [85]
138
H
H
H
29.6
i
Pr
143
H
H
34.49
i
Pr
i
Pr
i
Pr
140
33.63
i
Bu
i
Bu
i
Bu
142
33.53
260.8
(261.7)
139
Me
Me
Me
32.90;
32.82
(41.2)
26.8
26.6 [102]
259.1
141
Piv
Piv
Piv
32.84
144
CH
2
Ph
CH
2
Ph
CH
2
Ph
26.8
¼
(R
CH
2
Ph) is rationalized as the consequence of the dominant electronic stabilization
effect associated with greater delocalization and hence greater charge balance in the
phosphorus orbitals involved in the three-centre, four-electron H-P-N
ax
bond. This idea is
supported by X-ray: the short P-N
ax
bond distance in 138H
þ
is 2.078 A
.
Theoretical predictions have beenmade that ylides of phosphorus, nitrogen and sulfur are
potentially superstrong neutral organic bases. Limited experimental results: showed that the
Ph
3
P
¼
C(CH
3
)
2
ylide in THF was estimated to be in the range between 26 and 28 units [104].
General predictions are the following: phosphazenes and phosphorane can reach high
superbasicity levels of about 300 kcal mol
1
(for P7 or higher number of phosphorus atoms
in the system, n
¼
CH
2
ylide in DMSO has pK
a
¼
22.5 [103], while the pK
a
of (Ph(CH
3
)
2
N)
3
P
7), whereas the strongest organic phosphazene ylide superbases are
estimated to have (at n
5) gas phase basicities around or beyond 310-320 kcal mol
1
. The
phosphine superbases are predicted to have basicity comparable to P2 phosphazenes or P1
phosphorus ylides, whereas the respective proazaphosphatrane imines and ylides are
expected to be the strongest organic superbases which contain only a single phosphorus
atom.
Calculations have revealed that the proazaphosphatrane bases are approximately equal in
thermodynamic basicity to the Schwesinger P2 phosphazene bases (Table 2.14). The RHF/
6-31G* calculations of proton affinities indicate that phosphazene (Z
¼
NH) and phospho-
rus ylide (phosphorane, Z
CH
2
) counterparts (146 and 145) are stronger than the parent
Verkade superbase 139. Higher basicity is associated with the higher degree of delocaliza-
tion of the positive charge in the protonated iminophosphoranes and phosphorus ylides as a
result of the more electropositive character of phosphorus atom [67]. On the other hand,
phosphorus oxides 147 and 150 are weaker than 139, but still above the superbasicity
borderline. The strained polycycles 138, 149 and 148, as representatives of superbases
¼