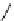Chemistry Reference
In-Depth Information
H
2
N
NH
2
NH
2
NH
H
2
N
NH
76
H
2
N
75
Figure 2.9
Structures of iminoamines 75-76
Functionally related to amidines are iminoamines, in which imino and amino func-
tionalities are placed at the termini of the conjugated backbone. Howard has published
high level calculations of the series of iminoamines employing B3LYP/6-31
G**//6-
31G** method [60]. These studies indicate that the larger the backbone, the larger the
conjugation (stabilization of the conjugate acid by the resonance mechanism) and
basicity. Theoretically predicted maximum gas phase PAs for linear (unbranched)
iminoamines with chain lengths up to six CH
þ
CH units slowly approached saturation
point at 266.3 kcal mol
1
. Single-branched iminoamines have PAs that are not much
larger, slowly approaching the saturation limit at 275.8 kcal mol
1
(iminoamine 75),
while double-branched iminoamines are more basic, with the saturation limit at
288.9 kcal mol
1
(molecule 76). Since an unsaturated carbon chain transmits conjugation
differently to an alternate
¼
structure there is a different relationship
between basicity and chain length (Figure 2.9).
¼
N
C
¼
N
C
¼
2.4 Guanidines
Guanidines have long been recognized as very strong nitrogen organic bases in solution.
The measured pK
a
(H
2
O) of guanidine is 13.6, while the most advanced G2 calculations
provide a PA of 235-236 kcal mol
1
. Furthermore, the biologically important compound
arginine has superbasic properties [61]. Guanidine is a stronger base than other nitrogen
compounds containing one potentially basic site (N-imino or N-amino) linked to a carbon
atom, for example, pyridines, amines, and amidines, and also those with two basic nitrogen
atoms, for example, diamines. Basic properties of guanidines have been used for catalysis of
various organic reactions [62]. The unusual thermodynamic stability of acyclic guanidines
and their monoprotonated forms is ascribed to the following factors: resonance stabiliza-
tion; Y-aromaticity [63,64]; favourable distribution of positive charge that leads to a
favorable Coulomb interaction; aromaticity; stabilization by intramolecular hydrogen
bonding; and the effect of solvation on the stability of the protonated form (strong hydrogen
bond between the cation and solvent molecules in solution). All of these factors play an
important role in the gas phase and in solution [65].
It was found experimentally that the protonation of guanidine occurs at the imino
nitrogen, as the amino nitrogen is less basic than the imino nitrogen atom by
30 kcal
mol
1
.