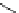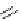Chemistry Reference
In-Depth Information
Table 2.6
Gas phase basicities of amidine derivatives [53,54]
Me
N
R
3
N
N
Me
Me
Me
Me
N
N
N
R
1
2
N
N
R
H
2
DBD
a
PMDBD
b
74
amidine
R
1
R
2
R
3
Type
c
GB
d
Base
GB
PA
278.0
e
65
VIN
>
258
66
VIN
254.85
71
Me
Me
(CH
2
)
3
NMe
2
AAM
246.1
242.9
256.7
72
Me
Me
1-Ad
AAM
244.0
240.4
248.2
DBU
CAM
243.4
239.7
247.5
DBD
CAM
242.5
amidine
Me
Et
t-Bu
AM
242.15
238.6
246.4
amidine
Me
H
(CH
2
)
3
NMe
2
FAM
241.7
238.0
251.7
PMDBD
CAM
241.15
237.6
251.7
(CH
2
)
2
N(2-py)
2
f
73
Me
H
FAM
241.1
DBN
CAM
241.0
237.4
245.1
amidine
Et
Me
n-Pr
EAM
240.75
237.3
245.0
amidine
Me
Me
(CH
2
)
2
OMe
AAM
240.55
236.9
248.3
amidine
Me
Ph
Me
BAM
239.7
236.2
244.0
amidine
Me
H
1-Ad
FAM
239.6
236.2
244.0
amidine
Me
Me
i-Pr
AAM
239.4
235.8
243.5
amidine
Me
Ph
Ph
BAM
235.5
74
CAM
233.65
amidine
Me
H
Me
FAM
232.0
a
1,5-Diazabicyclo[4.4.0]dec-5-ene.
b
PMDBD
¼
3,3,6,9,9-pentamethyl-2,10-diazabicyclo- [4.4.0]dec-1-ene.
c
VIN
¼
vinamidine, FAM
¼
formamidine, AAM
¼
acetamidine, AM
¼
amidine, BAM
¼
benzamidine, EAM
¼
Et
2
N-
acetamidine; CAM
cyclic amidine.
d
Re-evaluated values with different reference base.
e
B3LYP/6-31G* gas phase value [55].
f
py
¼
¼
pyridyl.
order: (CH
2
)
3
N(CH
3
)
2
>
(CH
2
)
2
OCH
3
. The basicity increase is associ-
ated with the formation of the resonance-assisted strong IMHB. The exceptionally high
basicity of 71 suggests that both basic sites participate in themonoprotonation reaction. One
site binds the proton and the other one interacts with the protonated function. The chelation
of a proton in flexible bidentate nitrogen ligands increases the gas phase basicity value by 5-
20 kcal mol
1
in comparison to monodentate bases. Strong basicity was also observed for
the flexible polyfunctional (2-pyridylethyl)-formamidine
(CH
2
)
2
N(CH
3
)
2
>
241.1 kcal mol
1
)
[58]. The separation of the two basic sites in 71 (and 74) by the alkane chain increases
the chelation effect against the proton. The size of IMHB effect depends on the strength
of the basic sites that can chelate the proton and the geometry of monocations, which
favours the formation of the IMHB. Direct azinyl substitution on the imino nitrogen in
formamidines, leads to proton chelation and IMHB, similar to 74. However, gas phase
basicity values are below the superbasicity threshold, due to less effective cation stabiliza-
tion by n-
73
(GB
¼
p
conjugation of the amidine with aza groups [59].