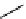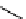Chemistry Reference
In-Depth Information
10.3.1.2
Plusbacins
Plusbacin A
1
(21) and the derivatives A
2
-A
4
and B
1
-B
4
are lipodepsipeptides isolated from
a strain numbered PB-6250 related to the genus Pseudomonas obtained from a soil sample
collected in the Okinawa Pref., Japan [56]. These compounds contain arginine residue and
lactone linkage with characteristic 3-hydroxy fatty acids [57] (Figure 10.6). Plusbacin A
3
(22) showed inhibitory activity against methicillin resistant Staphylococcus aureus [56,58].
Recent total synthesis of this compound was reported and the absolute configuration of the
lactone residue was determined as R [59].
10.3.1.3 Guadinomines
Guadinomines A and B (23-24) were recently isolated from the culture broth of Strepto-
myces sp. K01-0509 FERMBP-08504 strain by Omura et al. [60]. The structure contained
alanine and valine residues, 1,2-diamine and a cyclic guanidine. The absolute configuration
of the latter guanidine part was deduced from those of K01-0509B, isolated from the same
strain (Figure 10.7) [61]. These compounds possess activity towards a pathogenic Gram
negative bacterium in a type III secretion mechanism (IC
50
23
¼
0.01
g/mL, 24
¼
0.007
m
g/ml).
m
10.3.2 Natural Guanidines from Marine Invertebrates
10.3.2.1
Palau
amine
Palau
amine was isolated from a sponge, Stylotella agminata, collected in the Western
Caroline Islands. The structure was originally determined as 25 with hexacyclic bisgua-
nidine, in which bicyclo[3.3.0]azaoctane system (D and E) is cis-fused (at C11 and C12)
[62,63]. Recently the structure of palau
amine was revised from 25 to trans-fused 26 based
on synthetic studies of the compound with the structure in 25 [64], the computational
analysis of coupling constants of tetrabromostyloguanidine (27) [65] and further NMR
experiments of palau
amine-class metabolites [66,67]; the absolute configurationwas revised
from(12S,17R) to (12R,17S) [68] (Figure10.8).Palau
amine is lessnontoxic (LD
50
13mg/kg;
i.p. mice); it is quite active against P-388 and A549 (IC
50
0.1 and 0.2mg/mL, respec-
tively), less so against other cancer cell lines (HT-29 and KB), and possesses antibiotic
activity (against Staphylococcus aureus and Bacillus subtillis) and antifungal activity
(against Penicillium notatum).
Me
Me
R
OH
NH
2
O
O
O
N
OH
OH
N
H
N
H
2
N
H
2
N
NH
OH
O
NH
OH
NH
2
O e
O
HN
HN
K01-0509B
guadinomine A (
23
, R = OH)
guadinomine B (
24
, R = H)
Figure 10.7
Structures of guadinomine A and B (23-24) and K01-0509B