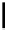Chemistry Reference
In-Depth Information
OH
O
PS-TBD
(1.8-3.0 equiv)
R
2
R
1
+
R
2
X
R
1
MeCN, 25 °C
O
O
O
COOEt
COMe
NO
2
CN
Cl
Br
Me
92% (64 h)
79% (22 h)
65% (168 h)
F
Me
Cl
N
O
O
F
O
Cl
Me
Me
Me
Cl
64% (24 h)
75% (1 h)
Scheme 6.11
Alkylation of phenols with alkyl halides
Xu et al. reported that a variety of phenols could be smoothly alkylated with alkyl halides
or activated aromatic fluorides in the presence of 1.8-3.0 equiv. of PS-TBD [45]
(Scheme 6.11).
The role of PS-TBD can be easily understood if it is considered that it works not only as a
base to deprotonate phenols, but also as a scavenger to trap unreacted excess starting
phenols. In addition, also established was a convenient strategy for performing multi-step
conversions in one-pot operation as represented by Scheme 6.12 [47].
Thus, the procedure consists of the following sequential treatments to derive the desired
double alkylated compounds: the first alkylation on a piperidine ring using R
1
X in the
presence of weakly basic PS-NMe
2
followed by addition of PS-NH
2
to sequester the
remaining excess R
1
X; the second alkylation on a pyrazole ring using R
2
X in the presence of
strongly basic PS-BEMP followed by addition of PS-NH
2
; and, finally, filtration and
concentration.