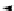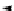Chemistry Reference
In-Depth Information
base 1 + base 2 + ----
[ super base ]
equation 1: unimetal super base
A
-
M
+
+ B
-
M
+
[ (A
-
+ B
-
) (M
+
)
2
]
equation 2: multimetal super base
A
-
M
1
+
+ B
-
M
2
+
[ (A
-
+ B
-
) (M
1
+
+
M
2
+
) ]
Scheme 1.1
Schematic equation for the definition of superbases proposed by Caubere
Computational calculation of their proton affinities indicates that these newgenerations are,
as expected, stronger than the original ones [12,18].
Organic chemists often use the words
as the intensive expression of
basic property; however, the criteria are ambiguous and dependent upon the chemists who
use the expression. Therefore, the expression such as
strong
or
super
is ambiguous and
causes confusion among organic chemists. Caubere has proposed the definition of
superbases as follows in his excellent review [19]: The term
strong
or
super
should only be
applied to bases resulting from a mixing of two (or more) bases leading to new basic species
possessing inherent new properties. The term
superbase
does not mean a base is
thermodynamically and/or kinetically stronger than another, instead it means that a basic
reagent is created by combining the characteristics of several different bases. The general
equation for the definition of a
superbases
superbase
is illustrated in Scheme 1.1, in which the
examples of
by
Schlosser [20] are given. Thus, the term superbases in general applies to ionic metal-
containing bases acting under irreversible proton abstraction.
One of important and beneficial characteristics of an organic base, especially from the
view point of environmental aspects, is the ability of recycling use in repeated reaction, in
which reversible proton transfer occurs between the base and a substrate, an acidic
counterpart. Thus, powerful organic bases that may be applicable in various organic
syntheses as base catalysts have attracted much attention. According to Caub
unimetal superbase
introduced by Caub
ere and a
multimetal superbase
s defini-
tion, organic superbases should be a mixture of two or more different kinds of amine species
and show a new property. In this topic nonionic powerful amine derivatives of amidines,
guanidines, phosphazenes and Verkade
ere
s bases with comparable or higher basicity to that of
DMAN are arbitrarily classified as organic superbases and discussed on their chemistry due
to basic characteristics, mainly focusing on their applications to organic synthesis as
potentially recyclable base catalysts. Related intelligent molecules are also discussed.
References
1. Haflinger, G. and Kuske, F.K.H. (1991) The Chemistry of Amidines and Imidates, Vol. 2 (eds S.
Patai and Z. Rapport), John Wiley & Sons, Chichester, pp. 1-100.
2. Raczynska, E.D., Maria, P.-C., Gal, J.-F. and Decouzon, M. (1994) Superbases in the gas phase.
Part II. Further extension of the basicity scale using acyclic and cyclic guanidines. Journal of
Physical Organic Chemistry, 7, 725-733.