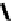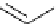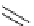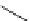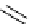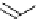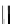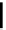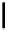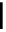Chemistry Reference
In-Depth Information
P
H
HN
NH
H
+
P
+
NH
NH
HN
NH
N
N
Verkade's base
Figure 1.6
Typical structure of Verkade
s base and its basicity due to trans-annular P-N
formation
Verkade [14] discovered proazaphosphatranes (cycloazaphosphines) as alternative
phosphorus-containing organobases, in which a P(III) atom bonded to three amino groups
is located at the bridge head. The basicity of Verkade
s bases is comparable to those of P2-
type phosphazene bases. The corresponding phosphonium salts formed by protonation on
the phosphorus atom are stabilized through effective trans-annular N
P bond formation, to
which the fourth nitrogen atom located at the alternative bridge head position participates;
this result in propellane-type compounds with tricylo[3.3.3]dodecane skeletons, as shown
in Figure 1.6.
In 1968, Alder [15] reported the preparation of 1,8-bis(dimethylamino)naphthalene
(DMAN) by N-methylation of 1,8-diaminonaphthalene. DMAN shows exceptional proton
affinity through bidentate-type coordination by the two dimethylamino groups located at
peri position of the naphthalene skeleton, in spite of being categorized as a weakly basic
aromatic amine (Figure 1.7). Thus, DMAN is called a
.
1,8-Bis(tetramethylguanidino)naphthalene (TMGN) [16] and guanidinophosphazenes
[17], such as tris[bis(dimethylamino)methylene]amino-N-tert-butylaminophosphorane
[(tmg)
3
N
t
Bu], are designed as hybrid organobases by the introduction of the guanidine
function into the proton sponge and phosphazene skeletons, respectively (Figure 1.8).
proton sponge
H
+
Me
2
N
NMe
2
Me
2
N
e
2
H
+
DMAN
Figure 1.7
Bidentate-type chelation of DMAN
N
t
Bu
PN
Me
2
N
NMe
2
N
Me
2
N
NMe
2
N
Me
2
N
N
N
NMe
2
Me
2
N
NMe
2
NMe
2
NMe
2
(tmg)
3
P=N
t
Bu
TMGN
Figure 1.8
Examples of hybrid organobases