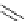Chemistry Reference
In-Depth Information
OMe
EtO
2
C
OMe
EtO
2
C
HO
OBn
t
Bu-P4 base (10 mol%)
O
OBn
F
TMS-NEt
2
(2 eq.), DMF
Br
Br
rt, 3 h, 94%
Me
Me
Me
Me
O
PdCl
2
(dppf) (3 mol%)
2M Na
2
CO
3
, dioxane
100
o
C, 24 h
X
B
O
X = OMe, H
BnO
EtO
2
C
Br
OMe
EtO
2
C
OMe
NBS
O
OBn
DMF, rt, 6 h
O
OBn
X
X
X = OMe (98%)
X = H (94%)
X = OMe (94%)
X = H (91%)
OBn
OBn
Pd(OAc)
2
(10 mol%)
PCy
3
(20 mol%)
K
2
CO
3
(2eq.), DMF
MW 100
o
C, 1 h
EtO
2
C
OMe
HO
2
C
OMe
O
OBn
O
OH
1) 10% Pd-C, H
2
(1 atm)
EtOAc-MeOH (1:1)
rt, 24 h
2) 1M NaOH, THF
rt, 3h
X
X
X = OMe (98%)
X = H (86%)
X = OMe (99%)
X = H (99%)
OBn
OH
Scheme 5.36
Synthesis of dictyomedins
5.3.2.3 Generation of Aryl anion from Arylsilane
Aryltrimethylsilanes have been used as important synthons and various desilylative
functionalizations have been investigated. Among these, anion mediated generation of
aryl anions is one of the most important methods for selective bond formation. However, the
reaction has been limited to aryltrimethylsilanes with strong electron-withdrawing groups
on the aromatic rings. It was found that
t
Bu-P4 base could be used as an excellent catalyst to