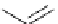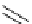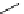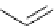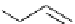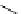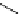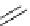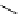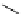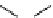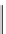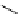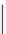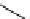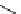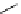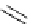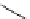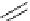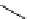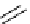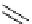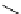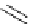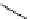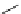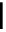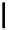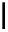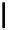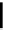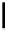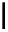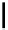Chemistry Reference
In-Depth Information
HO
O
F
t
Bu-P4 (10 mol%)
+
Et
3
SiH, DMF
NO
2
OMe
NO
2
OMe
rt, 33h
76 %
Scheme 5.35
Nucleophilic substitution using phenol derivative
development. Although they showed attractive biological activities in a preliminary biologi-
cal evaluation, the isolated sample amount was not sufficient enough for various biological
screening tests, because only a trace amount of dictyomedins can be isolated from a large
amount of a dried fruit body extract of Dictyostelium. So the synthetic supply of these
compounds was strongly desirable from the biological interest. Dictyomedins also have the
unique structural feature of a 4-aryldibenzofuran structure. Diaryl ether synthesis is consid-
ered to be one of the most important reactions in the synthetic plans [56] (Figure 5.6).
The syntheses of dictyomedins A and B were started with the coupling of ethyl 3-bromo-
4-fluorobenzoate and 3-benzyloxy-4-methoxyphenol. The reaction of the fluorobenzoate
with the phenol derivative proceeded smoothly at room temperature in the presence of two
equivalents of TMSNEt
2
as an additive and 10mol% of
t
Bu-P4 base to give the diaryl ether
in 94% yield. Subsequently, Suzuki-Miyaura coupling was carried out using two types of
boronic acid derivatives. 4-Benzyloxy-3-methoxyphenylboronic acid pinacolate and
4-benzyloxy phenylboronic acid pinacolate were easily prepared from vanillin or
4-bromophenol, respectively. The Suzuki coupling of the bromide with boronates was
carried out at 100
C for 24 h using PdCl
2
(dppf) and the desired arylated products were
obtained in high yields. In order to form dibenzofuran ring systemusing palladium catalysed
cyclization, the halogenation was first examined. The diaryl ethers were treated with NBS in
DMF at room temperature and the desired monobrominated products were obtained. Then,
palladiumcatalysed intramolecular biaryl formationwas investigated and the bromides were
treated with Pd(OAc)
2
and PCy
3
in the presence of potassium carbonate (K
2
CO
3
) at 100
C
for one hour under irradiation of microwave. The use of microwave dramatically accelerated
the cyclization and the irradiation was critical to obtain the products in high yields in a
shorter reaction time. The deprotection of benzyl groups was carried out by hydrogen (H
2
),
10% Pd/C and the consequent hydrolysis of the ethoxycarbonyl group afforded dictyome-
dins A and B in 98 and 86% yields, respectively [56] (Scheme 5.36).
HO
2
C
OMe
RO
2
C
X
OMe
A
B
O
OH
O
OR
C
Y
Y
OH
OR
Y=OMe Dictyomedin A
Y=H Dictyomedin B
Figure 5.6
Diarylether intermediate for dictyomedin synthesis