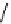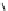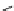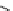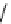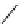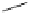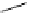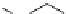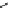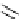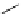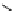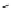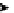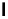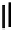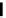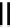Chemistry Reference
In-Depth Information
R
2
N
N
n
R
R
1
NN
R
3
NN
L
L
n
n
4
5
Figure 4.3
General structures of monocyclic 4 and bicyclic guanidines 5
4.2.1 Polysubstituted Acyclic and Monocyclic Guanidines
Schneck, in 1912, reported the synthesis of TMG (1) by the reaction of 1,1,3,3-tetramethyl-
2-methylthioamidinium salt 7, derived from the corresponding thiourea 6, and ammonia [8]
(Scheme 4.1). Later, the original method was improved using alternative reactions of
dimethylamine with cyanogen iodide and of 1,1-dimethyl-2-thiomethylamidine and di-
methylamine [9].
Chiral di-or trisubstituted acyclic guanidines [10] are, in the literature, prepared from the
corresponding amines through thiourea or carbodiimide intermediates (Scheme 4.2a); the
corresponding monocyclic guanidines could be supplied by application of these methods.
The cyano moiety of cyanogen bromide serves as a source of the core of guanidinyl
functions, when reacted with primary or secondary amines. Thus, N-cyanation reaction of
amines with cyanogen bromide was applied to the construction of not only acyclic [11] but
also monocyclic symmetrical systems [10b] (Scheme 4.2b).
Recently, polysubstituted guanidines were prepared in good yield by catalytic bismuth
(only 5mol%)-promoted synthesis through the guanidinylation reaction of N-benzoyl or
N-phenylthioureas with primary and secondary amines [12]. Furthermore, it was found that
half-sandwich rare earth metal complexes, which were prepared from [Ln(CH
2
Si-
Me
3
)
3
(thf)
2
] (Ln
Y, Yb, and Lu) and Me
2
Si(C
3
Me
4
H)NHR
0
(R
0
¼
Ph, 2,4,6-Me
3
C
6
H
2
,
t
Bu), serve as excellent catalyst precursors for the catalytic addition of various primary and
secondary amines to carbodiimides, efficiently yielding a series of guanidines [13]. A
possible mechanism for catalytic addition of secondary amine to carbodiimides is proposed
as shown in Scheme 4.3.
¼
4.2.2 Monosubstituted Guanidines (Guanidinylation)
Guanidine 8 with remote chiral centres, a monosubstituted acyclic guanidine, was prepared
by guanidinylation of an amine function with 3,5-dimethylpyrazole-1-carboxamidine [14]
I
-
S
SMe
MeI
NH
3
Me
Me
Me
Me
+
N
N
N
1
Me
Me
Me
Me
7
6
Scheme 4.1
Preparation of TMG (1) by Schenck