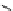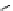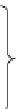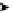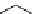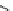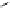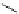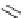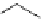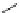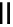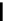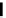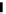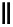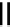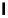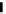Chemistry Reference
In-Depth Information
R
1
= R
2
= Me,
R
3
=
t
Bu (BTMG)
R
1
= R
2
= Et,
R
3
=
t
Bu
R
1
=
i
Pr,
R
2
= Et, R
3
=
t
Bu
R
1
= R
2
=
i
Pr, R
3
=
H
R
1
= R
2
=
i
Pr, R
3
=
Et
R
1
= R
2
=
i
Pr, R
3
=
t
Bu
R
1
= R
2
= R
3
=
i
Pr
R
3
H
N
N
N
R = H (TDB)
R = Me (MTDB)
R
2
R
1
Me
Me
N
Me
N
Me
N
R
2
N
R
1
N
R
N
1
2
3
Figure 4.1
Structures of TMG (1), Barton
s bases (2) and bicyclic guanidines 3
synthesis after modification of the guanidine skeleton to the chiral version according to
concept for the role of modified guanidines as chiral auxiliaries [5].
Preparation and use of supported TMG (1) as a novel base catalyst is discussed in a review
elsewhere [6]. Heterogeneous guanidines are provided as environmentally friendly base
catalysts and, thus, precise discussion on supported superbases is given in Chapter 6.
Guanidine chemistry has been excellently surveyed in topics [7]. This chapter focuses on
the synthetic utility of TMG (1) and its analogues in organic synthesis and application of
modified guanidine to asymmetric reactions.
4.2 Preparation of Chiral Guanidines
Guanidines are classified structurally into three types of compounds dependent upon
whether the guanidinyl function is incorporated into ring systems or not. Thus, in addition to
acyclic guanidines such as 1 and 2, monocyclic 4 and bicyclic guanidines 5 including 3 are
nominated as modified guanidines (Figure 4.3).
R
5
N
R
4
R
1
N
R
3
N
R
2
G
A-B
catalytic
reaction
stoichiometric
reaction
+
R
5
CD
A
N
E-B
B
-
B
A
R
4
R
1
+
N
R
3
N
R
2
+
C=D
E-F
G
·F-A
G
(ex)
substitution
(ex)
addition
active complex
A-B = acid or nucleophile
Figure 4.2
Classification of guanidine-participating reactions